2.2 Chemistry, Terminology, and Acronyms
This section focuses on chemistry, terminology, names, and acronyms for those PFAS most commonly reported in the environment, identified in scientific literature, and those PFAS most commonly tested for by current analytical methods. Other important classes of PFAS are introduced. This section also introduces the chemical manufacturing processes that influence the types of PFAS that are found in the environment.
The definition of PFAS continues to evolve to reflect continued study of these compounds and takes different forms depending on the regulatory body, operational criteria used, and the intended scope and application of the included list of chemicals. There is no universally accepted definition of PFAS. However, in general, PFAS are compounds characterized as having carbon atoms linked to each other and bonded to fluorine atoms at most or all of the available carbon bonding sites.
Table 2-1 provides examples of several definitions of PFAS (Hammel et al. 2022) developed by several authoritative agencies and researchers.
Table 2-1. Examples of PFAS definitions (Hammel et al. 2022, Table 2)(CC BY-NC-ND 4.0)
| Source of Definition | Year Defined | Definition | Additional Comments |
|---|---|---|---|
| Buck et al. | 2011 | “highly fluorinated aliphatic substances that contain one or more carbon (C) atoms on which all the hydrogen (H) substituents (present in the nonfluorinated analogues from which they are notionally derived) have been replaced by fluorine (F) atoms, in such a manner that they contain the perfluoroalkyl moiety CnF2n+1 –.” | Buck et al. (2011) is an open-access paper that provides a detailed explanation of PFAS terminology, classification, and origins, and recommends specific and descriptive terminology, names, and acronyms for PFAS. |
| OECD | 2021 | “PFASs, including perfluorocarbons, that contain a perfluoroalkyl moiety with three or more carbons (i.e. –CnF2n–, n ≥ 3) or aperfluoroalkylether moiety with two or more carbons (i.e.–CnF2nOCmF2m -, n and m ≥ 1).” | Updated in 2021 |
| OECD | 2021 | “fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (–CF3) or a perfluorinated methylene group (–CF2–) is a PFAS.” | OECD (2021) expanded the Buck et al.(2011) definition to include chemicals that contain the – CnF2n – moiety in addition to the CnF2n+1 – moiety, which encompasses chemicals with both ends of the carbon-fluorine chain connected to a hydrogen or a functional group, as well as cyclic analogs of linear PFAS. |
| Gluge et al. | 2020 | In addition to substances containing CnF2n+1, where n ≥ 1, it also “includes (i) substances where a perfluorocarbon chain is connected with functional groups on both ends, (ii) aromatic substances that have perfluoroalkyl moieties on the side chains, and (iii) fluorinated cycloaliphatic substances. Additionally, “polymeric PFAS with the –CF2– moiety and non-polymeric PFASwith the –CF2–CF2– moiety [excluding] non-polymericsubstances that only contain a –CF3 or –CF2– moiety, with the exception of perfluoroalkylethers and per- andpolyfluoroalkylether-based substances. For these two PFAS groups, substances with a –CF2OCF2– or –CF2OCFHCF– moietyare also included.” | Glüge et al. (2020) acknowledged the Buck et al. (2011) and OECD (2018) definitions while also considering the definition of PFAS to include:
|
| TURA Program, Massachusetts (TURA 2023) | 2023 | “Certain PFAS not otherwise listed includes those PFAS thatcontain a perfluoroalkyl moiety with three or more carbons (e.g., –CnF2n–, n ≥ 3; or CF3–CnF2n–, n ≥ 2) or a perfluoroalkylethermoiety with two or more carbons (e.g., –CnF2nOCmF2m_ or –CnF2nOCmFm, n and m ≥ 1), wherein for the example structures shown the dash (_) is not a bond to a hydrogen and may represent a straight or branched structure, that are not otherwise listed.” | |
| USEPA OPPT | 2021 | “.a structure that contains the unit R-CF2-CF(R’)(R”), where R, R’, and R” do not equal “H” and the carbon-carbon bond is saturated (note: branching, heteroatoms, and cyclic structures are included).” | Chemicals for review under TSCA to evaluate human health and environmental risks |
| NDAA, WA, CA, VT, ME | 2019, 2020, 2021 | Organic chemicals containing at least 1 fully fluorinated carbon atom | Authorities whose legislation defines PFAS as a class of fluorinated organic chemicals containing at least one fully fluorinatedcarbon atom. |
| USEPA CCL5 | “For the purposes of CCL 5, the structural definition of per- and polyfluoroalkyl substances (PFAS) includes chemicals that contain at least one of these three structures:R-(CF2)-CF(R′)R′′, where both the CF2 and CF moieties are saturated carbons, and none of the R groups can be hydrogenR-CF2OCF2-R′, where both the CF2 moieties are saturated carbons, and none of the R groups can be hydrogenCF3C(CF3)RR′, where all the carbons are saturated, and none of the R groups can be hydrogen.” | The USEPA CCL5 definition is less inclusive that the OECD definition, but it is more inclusive than the USEPA TSCA definition. |
The USEPA’s CompTox Chemicals Dashboard (https://comptox.epa.gov/dashboard/chemical-lists/PFASMASTER) provides a large publicly available resource for PFAS structures and predicted properties (Williams et al. 2017; Williams et al. 2022). This effort has taken two approaches to defining PFAS. The first, denoted PFASMASTER (USEPA 2020), was based on a simple join, or combination, of publicly available lists of PFAS chemicals reported by other entities, such as the OECD (2018) report. The second approach applied a small set of structure filters to the entire EPA DSSTox database (currently exceeding 1,200,000 substances), resulting in a list containing more than 8,000 PFAS structures, denoted PFASSTRUCTV3 (USEPA 2020); the most current version is PFASSTRUCTV5 (USEPA 2022) containing more than 14,000 structures. Please check USEPA’s CompTox Chemicals Dashboard for the most current version. The PFASMASTER file currently exceeds 12,000 substances and includes both PFAS structures (from PFASSTRUCV5) and structures and non-structurable chemicals (such as mixtures and polymers) from combining several public PFAS lists. Hence, the structure-based filters used in this effort expand the PFAS definition beyond the Buck et al. (2011) and OECD (2021) definitions and are designed to fully encompass publicly available PFAS lists, as well as to be inclusive of small, fluorinated chemicals of potential concern to USEPA. The most current PFASSTRUCTV5 uses a combination of four substructural filters (see Figure 2-3) and/or a threshold of 30% fluorine without hydrogen based on molecular formula count (not weight). The substructural filters (shown in Figure 2-3) are designed to be simple, reproducible and transparent, yet general enough to encompass the largest set of structures having sufficient levels of fluorination to potentially be considered PFAS. (USEPA 2022). 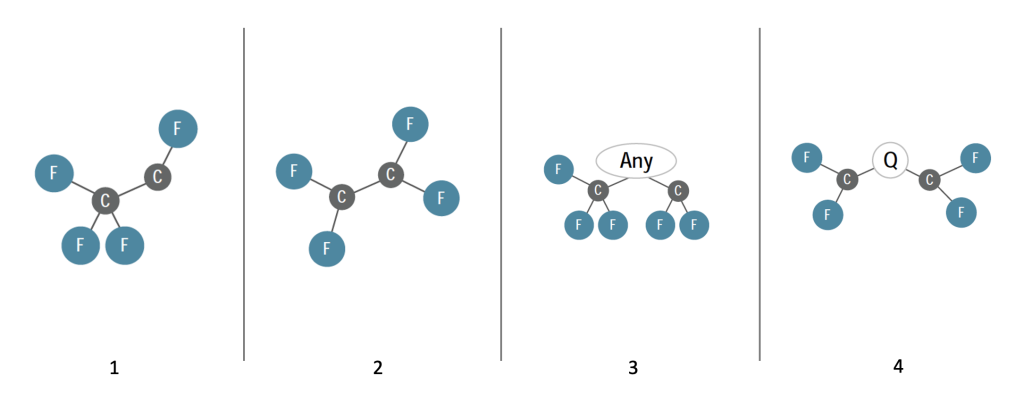
Figure 2-3. Four Substructural Filters of PFAS Used in Development of USEPA’s CompTox Dashboard for PFAS (USEPA 2022)
Note: Q can be any of the following atoms: B, O, N, P, S or Si. Source: Molecule figures M. Olson, Trihydro. Used with permission.
Whereas some PFAS definitions such as provided by USEPA’s CompTox Dashboard are designed to be broadly inclusive, definitions used in regulatory applications often must be more circumscribed and precisely worded. Examples of definitions used in regulatory applications include those used by USEPA TSCA and the USEPA Drinking Water Contaminant Candidate List 5 (CCL5); see Table 2.1. Other states may have their own definition of PFAS (see the Regulatory Programs Summary Table). For example, the state of Maine defines PFAS as substances that include any member of the class of fluorinated organic chemicals containing at least one fully fluorinated carbon atom (Maine State Legislature 2021).
General Concepts of Organofluorine Chemistry for PFAS
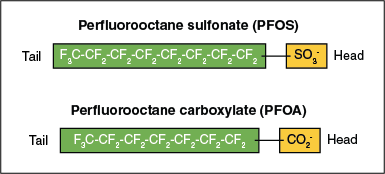
Organofluorine Chemistry:A branch of organic chemistry involving organic molecules with a carbon-fluorine bond. Organofluorine molecules have many commercial uses. They include PFAS, such as PFOA, shown below:
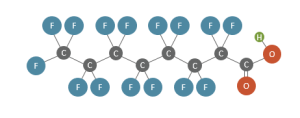
Example PFOA molecule, acid form
Source: M. Olson, Trihydro. Used with permission.
Gray spheres represent carbon atoms linked together in a chain; there are eight of them, so “octane” is used in the name. Blue spheres represent fluorine atoms bonded to carbon atoms. Red spheres represent oxygen atoms. Green sphere represents a hydrogen atom that dissolves away in water, which makes this an acid. Fluorine atoms are attached to all possible bonding sites, making this perfluorinated. If some of the fluorine atoms were replaced by other atoms (such as oxygen or hydrogen), it would be polyfluorinated. Without the hydrogen, the “head end” takes on a negative charge and can bond to things through electrostatic attraction. The fluorine “tail end” is strong and stable, giving it lipid- and water-repelling properties, but also making it persistent in the environment.
Isomer: A molecule with the same molecular formula as another molecule, but with a different chemical structure. Isomers contain the same number of atoms of each element, but have different arrangements of their atoms. See Figure 2-15 for an example; linear and branched PFOS contain the same number of carbon, fluorine, oxygen, and sulfur atoms, but these atoms are arranged differently depending on whether it is a linear or branched isomer of PFOS.
Homologue Groups and Homologous Series: A group of organic compounds, usually listed in order of increasing size, that has a similar structure (and therefore also similar properties) and whose structures differ only by the number of carbon atoms in the chain. For example, all of the linear and branched isomers of PFOS would be in the C8 homologue group, while all of the linear and branched isomers of perfluorohexane sulfonic acid (PFHxS) would be in the C6 homologue group. The C4-C12 PFSAs are a homologous series of perfluoroalkane sulfonates.
Throughout Section 2, the Buck et al. (2011) definition has been used for simplicity of discussion in the depiction of naming conventions of PFAS.
2.2.1 Naming Convention Considerations
There is confusion among the environmental community and the public due to overgeneralization when describing PFAS and the lack of consistent naming of specific PFAS. The use of consistent naming conventions would reduce confusion and support clearer communication (Buck et al. 2011; Wang et al. 2017).
Consistent naming also helps to distinguish PFAS from other organic compounds that contain fluorine. As defined in the literature, PFAS may include only one fluorinated aliphatic (carbon chain) substance. Alkyl/aliphatic groups are fully saturated carbon chains, which can be either cyclic, non-cyclic, branched or unbranched. Alkyl and aliphatic can be used interchangeably. Depending on the definition, PFAS may or may not include fluorinated compounds that contain aromatic (carbon ring) features in their structures (for example, active pharmaceutical ingredients, crop protection agents, or chlorofluorocarbons [refrigerants]) (Gaines, Sinclair, and Williams 2023; Glüge et al. 2020; Hammel et al. 2022). The inclusion of aromatic components in a chemical structure in the PFAS classification varies depending on what definition is applied. As Gaines, Sinclair, and Williams 2023 describes, including aromatics in a PFAS definition confounds things as the “PFAS” acronym includes the word “alkyl” that does not include aromatics. The authors go on to say that the “A” in PFAS could also be used to mean both aliphatic and aromatic, but this could also cause confusion as PFAS have not been used widely with this interpretation.
CAS numbers are another helpful tool for clearly identifying the chemical that is being referenced; however, care must be taken in selecting the correct CAS number to avoid confusion regarding the chemistry and behavior of the chemical being described. Some PFAS may exist in various ionic states, such as acids, anions (negatively charged), cations (positively charged), and zwitterions (both positively and negatively charged dipolar molecules), and each has its own CAS number (and some have no CAS number). The ionic state determines electrical charge and physical and chemical properties, which in turn control fate and transport in the environment (Section 5) and potential human health and ecological effects (Section 7). The ionic state of individual PFAS can result in significantly different physical and chemical properties (Section 4), such as solubility, volatility, and bioaccumulative potential.
International Union of Pure and Applied Chemistry (IUPAC) (https://iupac.org) nomenclature may also be used for identifying PFAS chemicals. The IUPAC nomenclature system is typically used in more formal literature such as patents. Examples of the IUPAC naming convention are provided below.
- PFOA: 2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-Pentadecafluorooctanoic acid
- PFOS: 1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctane-1-sulfonic acid
In the future, it may be necessary to expand the current naming conventions and acronym approaches to ensure that standardized naming is available for additional members of the PFAS family of compounds.
2.2.2 Introduction to the PFAS Family
PFAS are comprised of a wide variety of molecules with different physical and chemical properties and molecular weights with perfluoroalkyl moieties as common structural features, as note by Cousins et al. (2020), who identified specifically:
“…the PFAS class comprises distinct substances with very different structures and properties: high-molecular-weight polymers and high-molecular-weight non-polymers; neutral, anionic, cationic and zwitterionic substances; solids, liquids, and gases; highly reactive and non-reactive (inert) substances; soluble and insoluble substances; and volatile and involatile substances. In the environment, some PFAS are mobile and others immobile, and some bioaccumulate while others do not.”
This variety of PFAS with diverse properties is organized in the form of a PFAS family tree (Figure 2-4) that includes two primary classes: polymers and nonpolymers. Each class may contain many subclasses, groups, and subgroups, some of which are shown in the figure. This document focuses primarily on those nonpolymer PFAS most commonly detected in the environment and those PFAS that may be significant as “precursors” that can transform to more persistent forms. 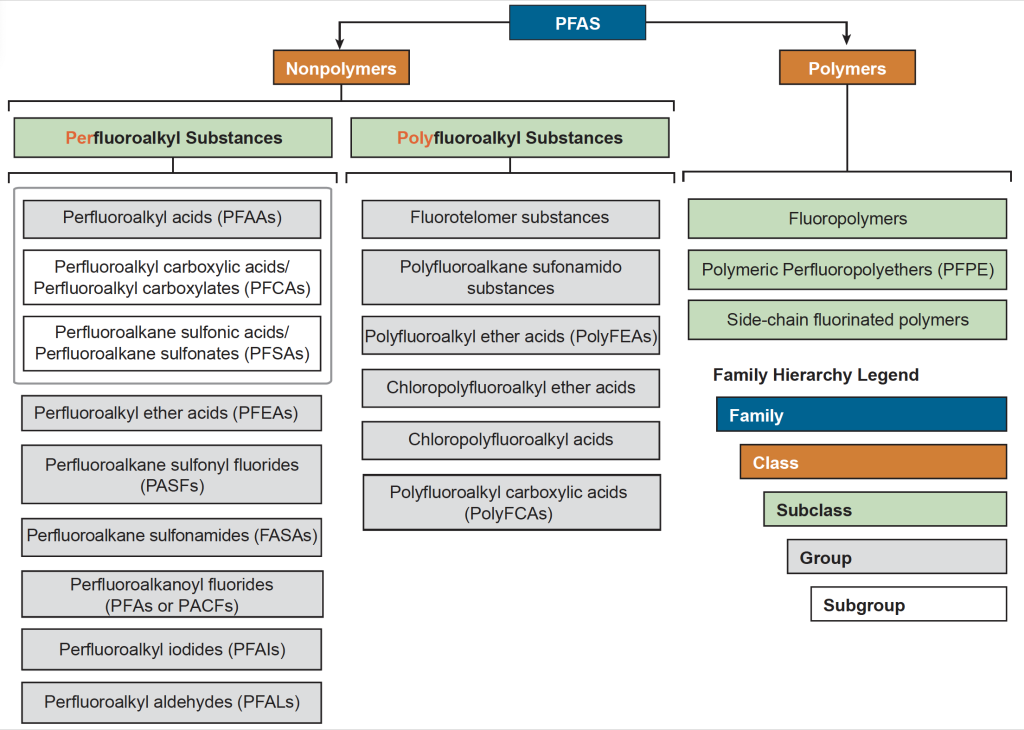
Figure 2-4. The PFAS family.
The family tree is further expanded in Figure 2-5, based on nomenclature provided in Buck et al. (2011), Organization for Economic Co-operation and Development OECD (2015), and Wang et al. (2017), with further introduction to some of these chemicals provided later in this section. Future updates to the family tree and nomenclature are expected to be necessary given the evolving public knowledge of these compounds. For example, other PFAS without analytical standards are being identified using nontarget analyses by research laboratories (Section 11). These PFAS do not necessarily have associated CAS numbers but are being identified by molecular structure. Naming conventions and categories of PFAS are developed as a means to communicate, manage, and address this class of many chemicals, which can include alternate naming conventions and a variety of rationales for assigning categories.
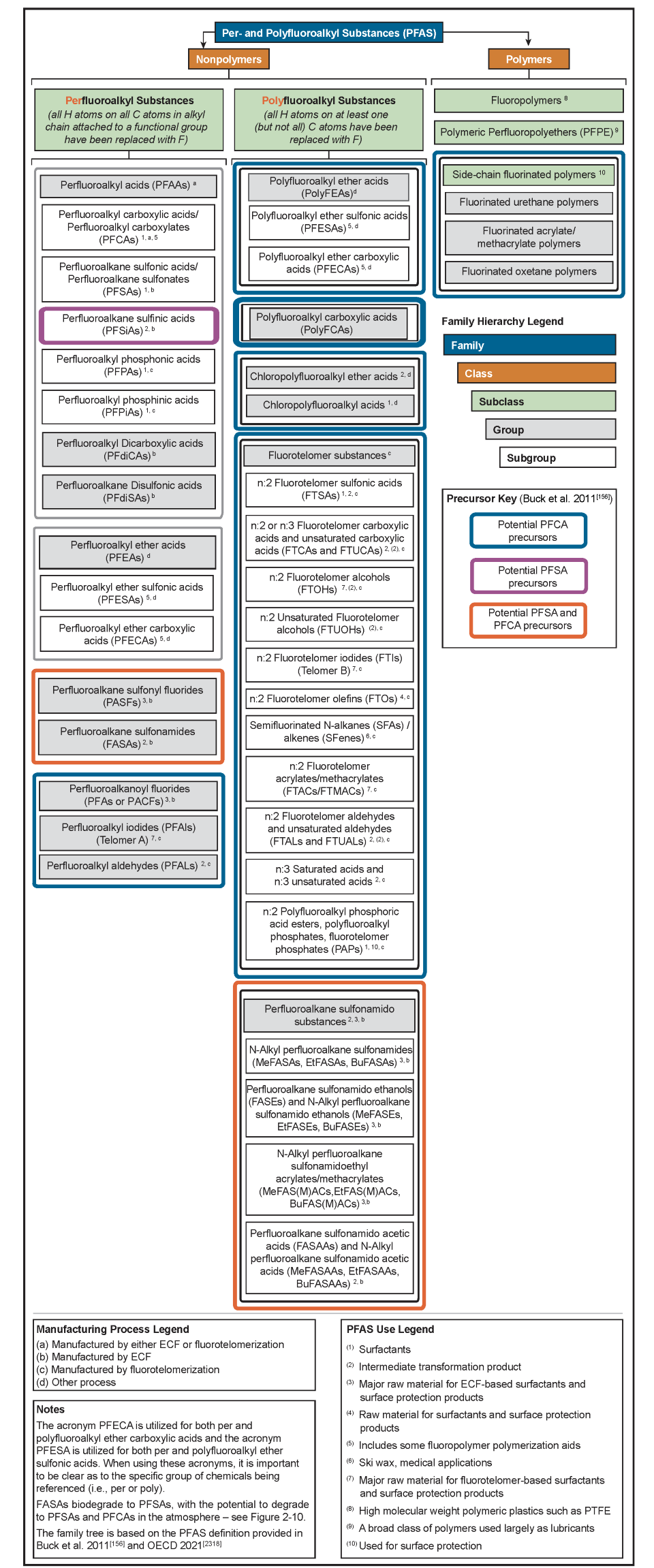
Figure 2-5. PFAS family tree. Adapted from a graphic provided courtesy of Paul Caprio, EA Engineering. A stand-alone PDF version of Figure 2-5 is available.
2.2.2.1 Polymer PFAS
Polymers are large molecules formed by combining many identical smaller molecules (or monomers, which are shorter chain molecules with no repeating units) in a repeating pattern.
The PFAS polymer class includes fluoropolymers, polymeric perfluoropolyethers, and side-chain fluorinated polymers (Henry et al. 2018; Buck et al. 2011; Wang et al. 2013):
- Fluoropolymers contain a carbon-only polymer backbone with fluorines directly attached to the carbon. Fluoropolymers include polymers like PTFE, ETFE, copolymer FEP, and PFA, which were historically made using processing aids ammonium perfluorooctanoate (APFO) or sodium perfluorooctanoate (NaPFO), which are salts of perfluorooctanoic acid (PFOA). Fluoropolymers also include polyvinylidene fluoride (PVDF), which was historically made using ammonium perfluorononanoate (APFN), the ammonium salt of perfluorononanoic acid (PFNA) (OECD 2015; Buck et al. 2011).
- The specific fluoropolymers PTFE, FEP, ETFE, and PFA have been referred to as “polymers of low concern” in two studies. Henry et al. (2018) notes that these specific fluoropolymers have high molecular weight and are extremely stable and PTFE has been demonstrated not to be bioavailable (Henry et al. 2018). Polymers of low concern are reported to pose little environmental or health risk once in a consumer product, and based on this, Henry et al. (2018) suggested that “polymers of low concern” should be considered separately from other PFAS when evaluating risk. An additional study (Korzeniowski et al. (2023)) describes the composition, uses, performance properties and functionalities of 14 fluoropolymers, including fluoroplastics and fluoroelastomers, and presents data to show that they satisfy the widely accepted polymer hazard assessment criteria to be considered polymers of low concern (PLC). Further, the study results show that fluoropolymers are a distinct and different group of PFAS and should not be grouped with other PFAS for hazard assessment or regulatory purposes.
- However, during the manufacture of some fluoropolymers, nonpolymer PFAS are used as processing aids and may be found as impurities in some fluoropolymer products (3M Company 1999; CalEPA 2018; see Section 5.4.5). For this reason, in order to prevent potential releases of nonpolymer PFAS processing aids (see AFPO and NaPFO discussion above) and short-chain polymeric byproducts/impurities, such as unreacted monomers, environmental controls are necessary during the manufacturing and further processing of fluoropolymers. However, it should be noted that another study (Lohmann et al. 2020) found that there was insufficient evidence to consider fluoropolymers as being of low concern for environmental and human health and that group of fluoropolymers should not be given a blanket exemption from regulatory review. According to Lohmann et al. (2020), the assessment and management of fluoropolymer products should consider the complete life cycle, including associated emissions during production and disposal.
- Polymeric perfluoropolyethers (PFPE) contain a carbon and oxygen polymer backbone with fluorines directly attached to carbon. Relatively little is known about these chemicals in the environment.
- Side-chain fluorinated polymers contain a nonfluorinated polymer backbone, off of which fluorinated side chains branch. These PFAS include fluorinated urethane polymers, fluorinated acrylate/methacrylate polymers, and fluorinated oxetane polymers. A few side-chain fluorinated polymers can become precursors for PFAAs, (Section 2.2.3.1) by transformation, when the point of connection of a fluorinated side chain on a polymer is broken to release a PFAA, or by release of fluorinated monomer residuals, or both.
2.2.2.2 Nonpolymer PFAS
Nonpolymer PFAS encompass two major subclasses: perfluoroalkyl substances and polyfluoroalkyl substances, which include many groups and subgroups of chemicals. Figure 2-6 provides general classification and chemical structures, examples of each group and/or subgroup, and examples of the primary uses of the nonpolymer PFAS highlighted in Figure 2-4 and Figure 2-5.
Nonpolymer PFAS were selected as the focus of this document because:
- they are included in most laboratory PFAS analyte lists (Section 11)
- they are the PFAS most commonly detected (to date) in humans, biota, and other environmental media and appear to be relatively more abundant at PFAS investigation sites (Section 6)
- data may be available regarding potential human health and ecological effects from environmental exposure for some of these chemicals (Section 7)
- state or federal standards or guidance values may exist or be under development for some of these chemicals (Section 8).
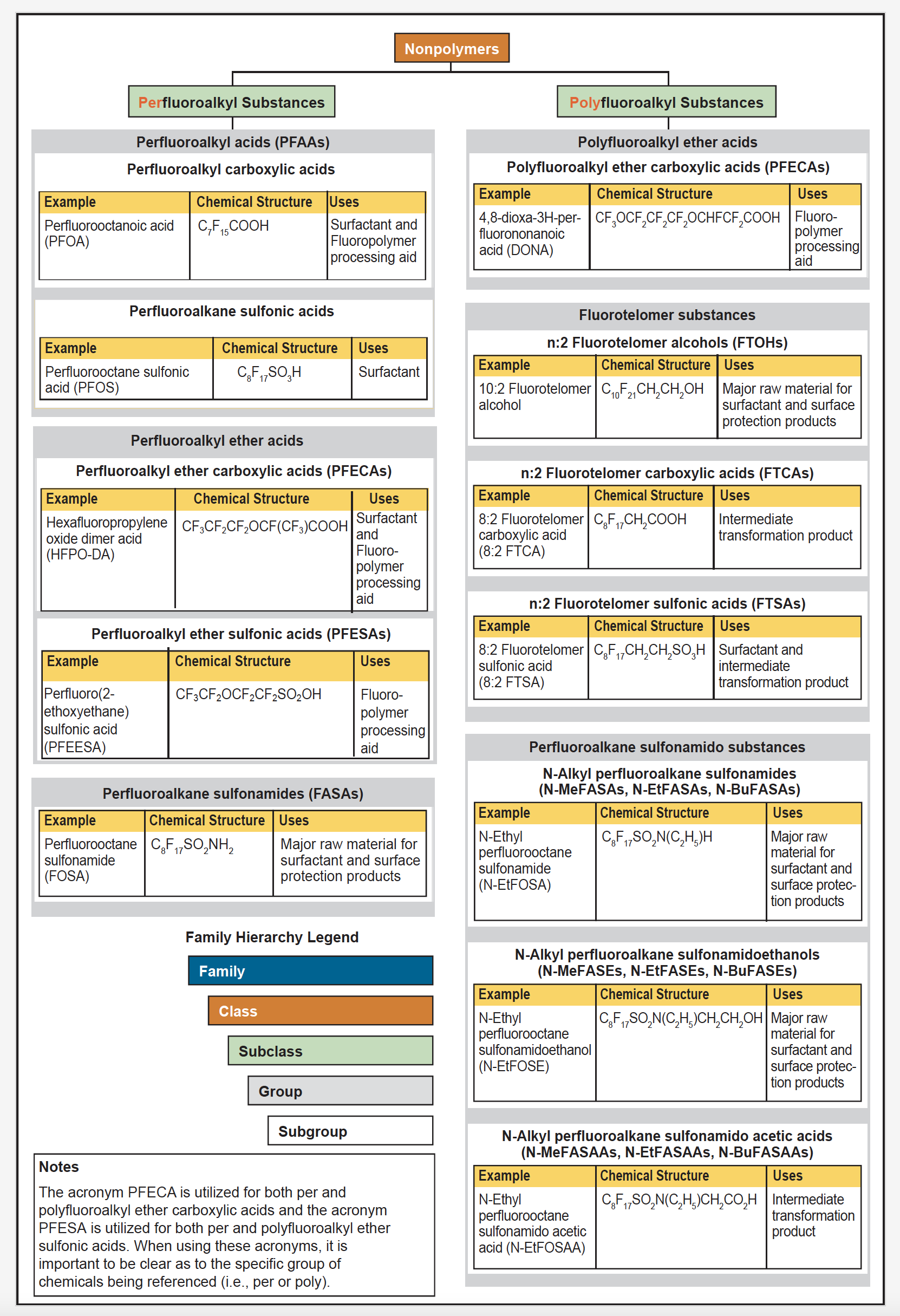
Figure 2-6. Examples of some nonpolymer PFAS subclasses discussed in this document.
Source: Adapted with permission from Buck, R.C., J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. de Voogt, A. A. Jensen, K. Kannan, S. A. Mabury, and S. P. van Leeuwenet. 2011. “Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins.” Integrated Environmental Assessment and Management, 7:513-541. Open access. Copyright 2011 SETAC. http://dx.doi.org/10.1002/ieam.258
2.2.3 Perfluoroalkyl Substances
Perfluoroalkyl substances are fully fluorinated alkane molecules that include (but are not limited to):
- perfluoroalkyl acids (PFAAs) and
- perfluoroalkane sulfonamides (FASAs).
The basic chemical structure is a chain (or tail) of two or more carbon atoms with a charged functional group (or head) attached at one end. The functional groups commonly are carboxylates or sulfonates, but other forms are also detected in the environment. Fluorine atoms are attached to all possible bonding sites along the carbon chain of the tail, except for one bonding site on the last carbon where the functional group head is attached. This structure, which is illustrated in Figure 2-7 for PFOS and PFOA, can be written as:
CnF2n+1-R
where “CnF2n+1” defines the length of the perfluoroalkyl chain tail, “n” is >2, and “R” represents the attached functional group head. Note that the functional group may contain one or more carbon atoms, which are included in the total number of carbons when naming the compound.
Figure 2-7. The tail and head structure of PFOS and PFOA molecules.
2.2.3.1 Perfluoroalkyl Acids (PFAAs)
PFAAs are some of the least complex PFAS molecules. They are essentially non-degradable under normal environmental conditions. Biotic and abiotic transformation of many polyfluoroalkyl substances may result in the formation of PFAAs. As a result, PFAAs are sometimes referred to as “terminal PFAS” or “terminal transformation products,” meaning no further transformation products will form from them under environmental conditions. Polyfluoroalkyl substances that transform to create terminal PFAAs are referred to as “precursors.” Longer chain PFAAs are not known to biotransform to shorter chain PFAAs.
The PFAA group is divided into two major subgroups (as shown in Table 2-1 and Figure 2-4).
- Perfluoroalkyl carboxylic acids (PFCAs), or perfluoroalkyl carboxylates, are used commercially and can be formed as terminal transformation products of select precursor polyfluoroalkyl substances, such as fluorotelomer alcohols (FTOHs). An example PFCA is PFOA.
- Perfluoroalkane sulfonic acids (PFSAs), or perfluoroalkane sulfonates, also are used commercially and can be formed as terminal transformation products of select precursor polyfluoroalkyl substances, such as perfluoroalkane sulfonamido ethanols (FASEs). An example PFSA is PFOS.
Other subgroups of PFAAs are introduced below. Some of those are compounds that are receiving increasing attention, are being added to commercial laboratory target analyte lists, and are being detected in the environment. Other PFAAs include:
- perfluoroalkane sulfinic acids (PFSiAs), associated with the electrochemical fluorination (ECF) process and also occurring as intermediate environmental transformation products
- perfluoroalkyl phosphonic acids (PFPAs) and phosphinic acids (PFPiAs), associated with the fluorotelomerization process and used as surfactants
PFAAs are the group of PFAS that make up the majority of PFAS typically included in commercial laboratory target analyte lists and are the primary PFAS for which federal or state health-based guidance values have been established. As a result, PFAAs tend to drive site investigation and remediation decisions, so it is helpful to understand the naming conventions for this class. Many of the commonly detected PFAAs are denoted using the following structural shorthand acronym:
PFXY where:
PF = perfluoro
X = the carbon chain length (using the same naming conventions as hydrocarbons based on the number of carbons (for example, B for butane or 4 carbons, Pe for pentane or 5 carbons)
Y = the functional group (for example, A = carboxylate or carboxylic acid and S = sulfonate or sulfonic acid)
Table 2-2 illustrates how this naming structure works for the PFCAs and PFSAs, which collectively are referred to as PFAAs.
Table 2-2. Basic naming structure and shorthand for PFAAs
| X | Y | Acronym | Name | Formula | CAS No.1 | DTXSID2 |
|---|---|---|---|---|---|---|
| B = buta (4 carbon) | A = carboxylate or carboxylic acid | PFBA | Perfluorobutanoate1 | C3F7CO2– | 45048-62-2 | 80892480 |
| Perfluorobutanoic acid1 | C3F7COOH | 375-22-4 | 4059916 | |||
| S = Sulfonate or sulfonic acid | PFBS | Perfluorobutane sulfonate | C4F9SO3– | 45187-15-3 | 60873015 | |
| Perfluorobutane sulfonic acid | C4F9SO3H | 375-73-5 | 5030030 | |||
| Pe = penta (5 carbon) | A = Carboxylate or carboxylic acid | PFPeA | Perfluoropentanoate | C4F9CO2– | 45167-47-3 | 00892487 |
| Perfluoropentanoic acid | C4F9COOH | 2706-90-3 | 6062599 | |||
| S = Sulfonate or sulfonic acid | PFPeS | Perfluoropentane sulfonate | C5F11SO3– | 175905-36-9 | 70892479 | |
| Perfluoropentane sulfonic acid | C5F11SO3H | 2706-91-4 | 8062600 | |||
| Hx = hexa (6 carbon) | A = Carboxylate or carboxylic acid | PFHxA | Perfluorohexanoate | C5F11CO2– | 92612-52-7 | 20892484 |
| Perfluorohexanoic acid | C5F11COOH | 307-24-4 | 3031862 | |||
| S = Sulfonate or sulfonic acid | PFHxS | Perfluorohexane sulfonate | C6F13SO3– | 108427-53-8 | 80873012 | |
| Perfluorohexane sulfonic acid | C6F13SO3H | 355-46-4 | 7040150 | |||
| Hp = hepta (7 carbon) | A = Carboxylate or carboxylic acid | PFHpA | Perfluoroheptanoate | C6F13CO2– | 120885-29-2 | 60892483 |
| Perfluoroheptanoic acid | C6F13COOH | 375-85-9 | 1037303 | |||
| S = Sulfonate or sulfonic acid | PFHpS | Perfluoroheptane sulfonate | C7F15SO3– | 146689-46-5 | 8059920 | |
| Perfluoroheptane sulfonic acid | C7F15SO3H | 375-92-8 | 20892505 | |||
| O = octa (8 carbon) | A = Carboxylate or carboxylic acid | PFOA | Perfluorooctanoate | C7F15CO2– | 45285-51-6 | 40892486 |
| Perfluorooctanoic acid | C7F15COOH | 335-67-1 | 8031865 | |||
| S = Sulfonate or sulfonic acid | PFOS | Perfluorooctane sulfonate | C8F17SO3– | 45298-90-6 | 80108992 | |
| Perfluorooctane sulfonic acid | C8F17SO3H | 1763-23-1 | 3031864 | |||
| N = nona (9 carbon) | A = Carboxylate or carboxylic acid | PFNA | Perfluorononanoate | C8F17CO2– | 72007-68-2 | 80892485 |
| Perfluorononanoic acid | C8F17COOH | 375-95-1 | 8031863 | |||
| S = Sulfonate or sulfonic acid | PFNS | Perfluorononane sulfonate | C9F19SO3– | 474511-07-4 | 60873010 | |
| Perfluorononane sulfonic acid | C9F19SO3H | 68259-12-1 | 8071356 | |||
| D = deca (10 carbon) | A = Carboxylate or carboxylic acid | PFDA | Perfluorodecanoate | C9F19CO2– | 73829-36-4 | 40892481 |
| Perfluorodecanoic acid | C9F19COOH | 335-76-2 | 3031860 | |||
| S = Sulfonate or sulfonic acid | PFDS | Perfluorodecane sulfonate | C10F21SO3– | 126105-34-8 | 00873014 | |
| Perfluorodecane sulfonic acid | C10F21SO3H | 335-77-3 | 3040148 | |||
| Un = undeca (11 carbon) | A = Carboxylate or carboxylic acid | PFUnA or PFUnDA | Perfluoroundecanoate | C10F21CO2– | 196859-54-8 | 30892475 |
| Perfluoroundecanoic acid | C10F21COOH | 2058-94-8 | 8047553 | |||
| S = Sulfonate or sulfonic acid | PFUnS PFUnDS | Perfluoroundecane sulfonate | C11F23SO3– | 441296-91-9 | 40904578 | |
| Perfluoroundecane sulfonic acid | C11F23SO3H | 749786-16-1 | 40904573 | |||
| DoD = dodeca (12 carbon) | A = Carboxylate or carboxylic acid | PFDoDA | Perfluorododecanoate | C11F23CO2– | 171978-95-3 | 00892482 |
| Perfluorododecanoic acid | C11F23COOH | 307-55-1 | 8031861 | |||
| S = Sulfonate or sulfonic acid | PFDoDS | Perfluorododecane sulfonate | C12F25SO3– | 343629-43-6 | 00904574 | |
| Perfluorododecane sulfonic acid | C12F25SO3H | 79780-39-5 | 20873011 | |||
| TrD = trideca (13 carbon) | A = Carboxylate or carboxylic acid | PFTrDA | Perfluorotridecanoate | C12F25CO2– | 862374-87-6 | 20892489 |
| Perfluorotridecanoic acid | C12F25COOH | 72629-94-8 | 90868151 | |||
| S = Sulfonate or sulfonic acid | PFTrDS | Perfluorotridecane sulfonate | C13F27SO3– | NA | 00904579 | |
| Perfluorotridecane sulfonic acid | C13F27SO3H | 791563-89-8 | 20904576 | |||
| TeD = tetradeca (14 carbon) | A = Carboxylate or carboxylic acid | PFTeDA | Perfluorotetradecanoate | C13F27CO2– | 365971-87-5 | 60892488 |
| Perfluorotetradecanoic acid | C13F27COOH | 376-06-7 | 3059921 | |||
| S = Sulfonate or sulfonic acid | PFTeDS | Perfluorotetradecane sulfonate | C14F29SO3– | 343629-46-9 | 30904582 | |
| Perfluorotetradecane sulfonic acid | C14F29SO3H | 1379460-39-5 | 80904577 | |||
| NA = not available 1Older nomenclature may use butyrate or butyric acid. 2 Link to DTXSID: https://comptox.epa.gov/dashboard/chemical-lists/PFASMASTER (helpful to use as a means of finding structural depictions and availability of other public data) |
||||||
Note that for PFCAs, the total number of carbons used for naming the compound includes the carbon in the carboxylic acid functional group (COOH). For example, although PFOA has seven carbons in its fluoroalkyl tail, all eight of the carbons in the molecule are used to name it, hence perfluorooctanoate. But in terms of chemical behavior, PFOA would be more analogous to seven-carbon perfluoroheptane sulfonate (PFHpS) than to eight-carbon perfluorooctane sulfonate (PFOS).
Table 2-2 shows the PFAA names and formulas in both the anionic (also referred to as “deprotonated” or negatively charged) and acid (also referred to as protonated or neutral) forms. The anionic form is the state in which PFAAs are found in the environment, except in very rare situations (for example, extremely low pH). The anionic and acid forms of PFAA names are often incorrectly used interchangeably (for example, perfluorooctane sulfonate and perfluorooctane sulfonic acid), and the same acronym (in this case, PFOS) applies to both forms. However, as discussed below and in Section 4, their physical and chemical properties are different, and it is important to know which form is being described.
Until recently, PFCAs and PFSAs have been the subgroups most commonly tested for in the environment; however, a wide range of PFAS with other functional groups exists for which the same “PFXY” shorthand shown above may or may not apply. For naming conventions for these compounds, please refer to Buck et al. (2011).
Long-Chain Versus Short-Chain Distinction
PFAS, predominantly PFAAs, are sometimes described as long-chain and short-chain as a shorthand way to categorize PFCAs and PFSAs that may behave similarly in the environment; however, it is important not to generalize about PFAA behavior based only on chain length. As recent research suggests, other factors besides chain length may affect bioaccumulation potential of PFAS (Ng and Hungerbühler 2014).
According to the OECD (2013):
- Long-chain refers to:
- PFCAs with eight or more carbons (seven or more carbons are perfluorinated)
- PFSAs with six or more carbons (six or more carbons are perfluorinated)
- Short-chain refers to:
- PFCAs with seven or fewer carbons (six or fewer carbons are perfluorinated)
- PFSAs with five or fewer carbons (five or fewer carbons are perfluorinated)
Table 2-3 illustrates the differences in the short-chain and long-chain PFCAs and PFSAs.
Table 2-3. Short-chain and long-chain PFCAs and PFSAs
| Number of Carbons | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| PFCAs | Short-chain PFCAs | Long-chain PFCAs | |||||||
| PFBA | PFPeA | PFHxA | PFHpA | PFOA | PFNA | PFDA | PFUnA | PFDoA | |
| PFSAs | PFBS | PFPeS | PFHxS | PFHpS | PFOS | PFNS | PFDS | PFUnS | PFDoS |
| Short-chain PFSAs | Long-chain PFSAs | ||||||||
Anions Versus Acids
As noted above, the names for the anionic and acid forms of PFAAs are often used interchangeably, but it is critical to know which form is being discussed because of differences in their physical and chemical properties and behavior in the environment (Section 6). Some important things to keep in mind regarding the anionic versus acid forms are:
- Most PFAAs are present in environmental and human matrices in their anionic form. For example, PFOS is present in the environment in the anionic form, perfluorooctane sulfonate.
- Although laboratories may be reporting PFOA or PFOS using the acid form of their name, they are actually measuring the anionic form (for example, perfluorooctanoate or perfluorooctane sulfonate), as this is the form that exists in the environment.
- The acid form and their associated cationic salts have CAS numbers, while the anionic forms may not (Table 2-2). For example, PFOS can exist as different salts (cationic), including sodium, lithium, potassium, or ammonium. Each of these salts will have a different CAS number:
- PFOS, acid form CAS No.: 1763-23-1
- PFOS, potassium salt CAS No.: 2795-39-3
- PFOS, ammonium salt CAS No.: 29081-56-9
- When the salt or acid exists in water or other liquids, it will dissociate (lose its hydrogen or associated ion), and the salt or acid will break off and form the anion (for example, COO– or SO3–). Figure 2-8 illustrates the dissociation of PFBA.
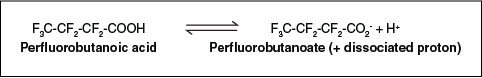
Figure 2-8. Dissociation of PFBA.
It is most important to distinguish between the acid form and anionic form when reporting the physical and chemical properties. The discussion of PFAS properties in this guidance document generally refers to the anionic form; it will be specifically called out if the acid form is being discussed.
A Note About PFAS Naming in Laboratory Reports (see Section 11)
Even though PFAAs occur as anions in the environment, some laboratories report all of their results in the acidic form, while others may report PFCAs as acids (for example, perfluorooctanoic acid) and PFSAs as anions (for example, perfluorooctane sulfonate). Different naming conventions in laboratory reports have led to confusion regarding exactly which form of the PFAA the labs are measuring. Although the lab is measuring the concentration of PFAA anions present in the sample, where the results are reported as an acid, the lab has adjusted for the H+ cation (which has so little mass, this does not affect the resulting concentration). It should be noted that the standards used by laboratories to perform analyses may be prepared from PFAA salts, as is often the case for sulfonate standards. If so, the lab must adjust the reported concentration to account for the mass of the counterion (typically Na+ or K+). The calculation to do this is described in section 7.2.3 of EPA Method 537 (USEPA 2009[794]).
2.2.3.2 Perfluoroalkane Sulfonamides (FASAs)
FASAs, such as perfluorooctane sulfonamide (FOSA), are products and/or intermediates from the ECF process for surfactants and surface protection products. FASAs can transform to form PFAAs such as PFOS.
2.2.3.3 Other Perfluoroalkyl Substances
Other perfluoroalkyl substances shown on Figure 2-4 and Figure 2-5 include:
- perfluoroalkane sulfonyl fluorides [PASFs, such as perfluorooctane sulfonyl fluoride (POSF) and perfluorobutane sulfonyl fluoride (PBSF)], and perfluoroalkanoyl fluorides (PAFs), associated with the ECF process
- perfluoroalkyl iodides (PFAIs) and perfluoroalkane aldehydes (PFALs), associated with the fluorotelomerization process
- perfluoroalkyl ether acids, including perfluoroalkyl ether carboxylic acids (PFECAs) and perfluoroalkyl ether sulfonic acids (PFESAs)
As discussed in Section 2.4, some PFECAs have been developed or used as replacements for other PFAS that are phased out of production and use. This includes GenX chemicals (see text box). Other emerging fluorinated replacement PFECAs more recently detected in the environment, such as perfluoro-2-methoxyacetic acid (PFMOAA), are described in Sun et al. (2016).
- hexafluoropropylene oxide (HFPO) dimer acid (HFPO-DA, CAS No. 13252-13-6, also known as 2,3,3,3-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)propanoic acid [PFPrOPrA] or FRD-903) and
- its ammonium salt (ammonium, 2,3,3,3- tetrafluoro-2-(heptafluoropropoxy) propanoate [CF3CF2CF2OCF(CF3)COO–NH4+, CAS No. 62037-80-3, also known as FRD -902])
(Wang et al. 2013; Buck 2015; USEPA 2018).
Prior to their use in PTFE production, GenX chemicals were produced as a byproduct of other manufacturing processes (NC DEQ 2018). From the GenX Toxicity Assessment document by USEPA (2022), HFPO also is used to manufacture other HFPO-DA derivatives, fluoropolymers and perfluoropolyethers, and other specialty agrochemical, semiconductor, and pharmaceutical applications. HFPO-trimer acid and longer polymer fluorides can be formed from reaction of HFPO-DA.
Further discussion of the GenX chemicals is provided in Section 2.4.6. The chemical structure of the ammonium salt is shown in Figure 2-9.
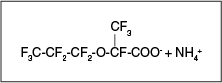
Figure 2-9. Example replacement chemistry structure for GenX Ammonium Salt.
In addition to linear and branched structures, certain cyclic structures have much in common with the noncyclic PFAS and are consistent with the definitions/descriptions provided above. As an example, Figure 2-10 illustrates the structure of perfluoro-4-ethylcyclohexanesulfonate, also perfluoro-p-ethylcyclohexylsulfonic acid (PFECHS), which some classify as a PFAS (MPART 2020). It is used in airplane hydraulic fluids and has been found both in the environment (Kaboré et al. 2018; Howard and Muir 2010; De Silva et al. 2011; Lescord et al. 2015; Houde et al. 2016) and in human blood (Miaz et al. 2020). The characteristics of PFECHS include:
- fully fluorinated six-carbon ring
- nonaromatic
- sulfonate active group
- perfluorinated two-carbon tail
PFECHS fits the Buck et al. (2011) description by having a fully fluorinated aliphatic tail of one or more carbon atoms attached to a charged functional group head.
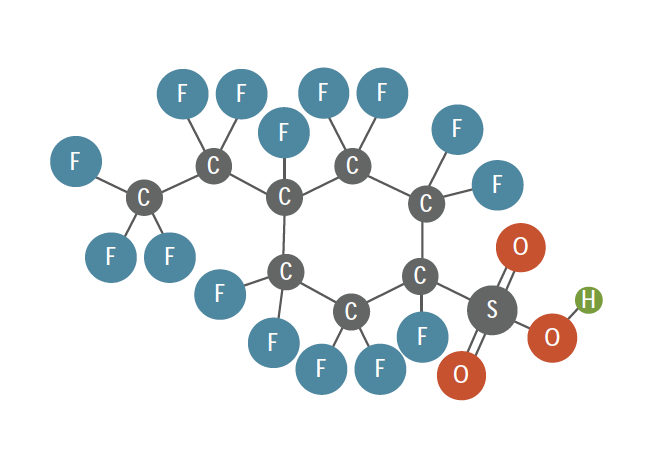
Figure 2-10. Illustration of perfluoro-p-ethylcyclohexyl sulfonic acid structure.
Source: M.Olson, Trihydro. Used with permission.
2.2.4 Polyfluoroalkyl Substances
Polyfluoroalkyl substances including some side-chain fluorinated polymers are increasingly being identified as important to understanding the occurrence, fate, and transport of PFAS at release sites and in the environment (OECD 2013; Butt, Muir, and Mabury 2014; Liu and Mejia-Avendaño 2013; Wang et al. 2011; Mejia-Avendaño et al. 2020). Figure 2-4 and Figure 2-5 highlight the groups of polyfluoroalkyl substances that, to date, have most commonly been detected at PFAS sites (see Barzen-Hanson et al. 2017; OECD 2018). Of the approximately 4,700 PFAS identified in the OECD (2018) study, about 90% were potential precursors to PFAAs.
Polyfluoroalkyl substances are distinguished from perfluoroalkyl substances by not being fully fluorinated. Instead, they are aliphatic substances for which all hydrogen atoms attached to at least one (but not all) carbon atoms have been replaced by fluorine atoms, in such a manner that they contain the perfluoroalkyl moiety CnF2n+1 (Buck et al. 2011), see Figure 2-6.
The carbon-hydrogen (or other nonfluorinated) bond in polyfluoroalkyl molecules creates a “weak” point in the carbon chain that may be susceptible to biotic or abiotic transformation. As a result, many polyfluoroalkyl substances that contain a perfluoroalkyl CnF2n+1 moiety are potential precursor compounds that have the potential to be transformed into PFAAs.
Figure 2-11 provides examples of transformation pathways for environmentally relevant polyfluoroalkyl precursors derived from two PFAS production methods, fluorotelomerization and ECF, respectively (Buck et al. 2011; Liu and Mejia-Avendaño 2013; Butt, Muir, and Mabury 2014; Martin et al. 2006). Note that these figures include some PFAS not discussed in this guidance document, but described in Buck et al. (2011). Refer to Section 5.4 for further information on transformation processes, noting that not all transformation products will be formed through every environmental transformation process.
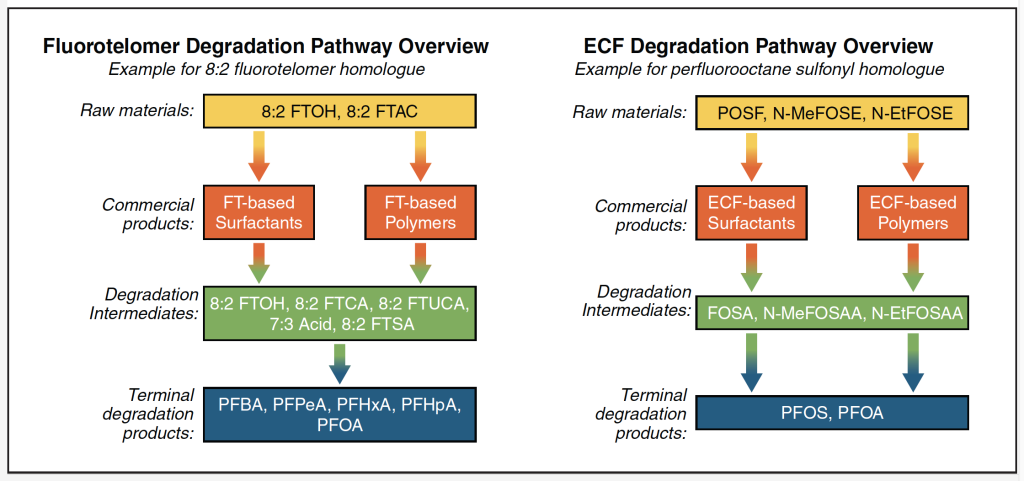
Figure 2-11. Example polyfluoroalkyl substance transformation pathways.
(Note that transformation of POSF-based products is for the terrestrial environment, but transformation into lower homologues of PFCAs and PFSAs in the atmosphere is also possible, see references in the paragraph above.)
2.2.4.1 Fluorotelomer Substances
Fluorotelomer substances are polyfluoroalkyl substances produced by the fluorotelomerization process. As shown in Figure 2-11, the transformation of fluorotelomer-based substances is a potential source of PFCAs in the environment, but not PFSAs (Buck et al. 2011).
Fluorotelomer-based polyfluoroalkyl substances are named using an “n:x” prefix where “n” indicates the number of fully fluorinated carbon atoms (n > 2) and “x” indicates the number of carbon atoms that are not fully fluorinated (x > 1). An example of a polyfluoroalkyl substance is shown in Figure 2-12, which also illustrates the “n:x” naming convention.
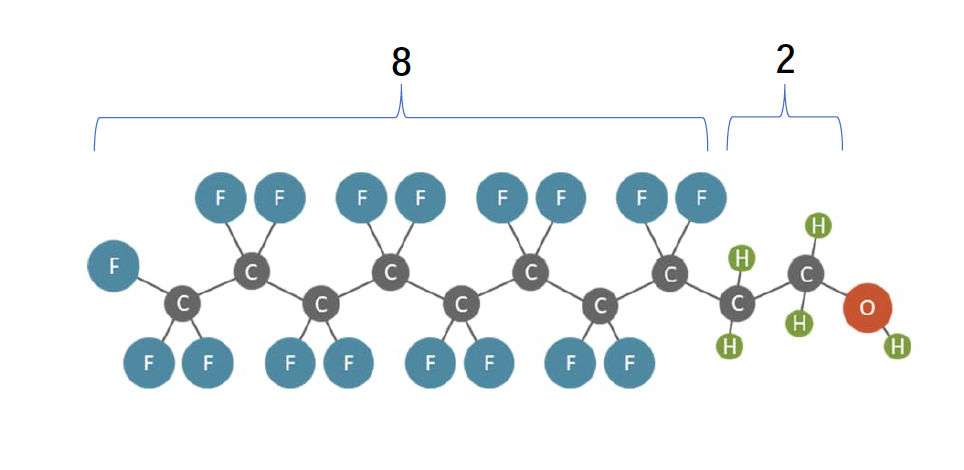
Figure 2-12. Example of a polyfluoroalkyl substance.
Source: M. Olson, Trihydro. Used with permission.
Some of the fluorotelomer substances most commonly detected in the environment to date are as follows (Section 6):
- Fluorotelomer alcohols (FTOH): The n:2 fluorotelomer alcohols (n:2 FTOHs) are key raw materials in the production of n:2 fluorotelomer acrylates and n:2 fluorotelomer methacrylates (Buck et al. 2011).
- Fluorotelomer sulfonic acids (FTS): The n:2 fluorotelomer sulfonic acids (n:2 FTSs) have been detected in environmental matrices at sites where aqueous film-forming foam (AFFF) has been used, and also in wastewater treatment plant effluents and landfill leachate. FTSs are precursor compounds and can undergo aerobic biotransformation to form PFCAs (Buck et al. 2011).
- Fluorotelomer carboxylic acids (FTCA): These compounds form through the biotransformation of FTOHs (Figure 2-11; (Buck et al. 2011; Mejia-Avendaño et al. 2016) and have been detected in landfill leachate. Note that the –COOH functional group on these fluorotelomer compounds means they may have either an even or odd number of carbons, so they may have n:2 or n:3 prefixes.
Other fluorotelomer (FT) substances are shown in Figure 2-6.
2.2.4.2 Perfluoroalkane Sulfonamido Substances
The subgroups of perfluoroalkane sulfonamido substances shown in Figure 2-5 and discussed below have been detected in the environment and humans (Buck et al. 2011). Perfluoroalkane refers to the fully fluorinated carbon chain tail, but these compounds also contain one or more CH2 groups in the head of the molecule attached to the sulfonamido spacer (Figure 2-13). They are either used as raw materials for surfactant and surface treatment products, or they are present as intermediate transformation products of these raw materials. As shown in the transformation pathways in Figure 2-11, some perfluoroalkane sulfonamido substances have been found to transform to PFOS (Mejia-Avendaño and Liu 2015). Environmentally relevant perfluoroalkane sulfonamido substances include:
- N-Alkyl perfluoroalkane sulfonamides (N-alkyl FASAs) are raw materials used for surfactant and surface treatment products that include N-methyl perfluorooctane sulfonamide (N-MeFOSA) and N-ethyl perfluorooctane sulfonamide (N-EtFOSA) (Buck et al. 2011)
- Perfluoroalkane sulfonamido ethanols (FASEs) and N-alkyl perfluoroalkane sulfonamido ethanols (N-MeFASEs, N-EtFASEs, N-BuFASEs) are raw materials for surfactant and surface treatment products (Buck et al. 2011). Figure 2-13 illustrates the structure of N-EtFOSE
- Perfluoroalkane sulfonamido acetic acids (FASAAs) and N-alkyl perfluoroalkane sulfonamido acetic acids (N-MeFASAAs, N-EtFASAAs, N-BuFASAAs) are intermediate transformation products of FASEs, N-MeFASEs, N-EtFASEs, and N-BuFASEs (Figure 2-11) (Buck et al. 2011)
- N-alkyl perfluoroalkane sulfonamidoethyl acrylates/methacrylates (N-MeFAS(M)ACs, N-EtFAS(M)ACs, N-BuFAS(M)ACs)
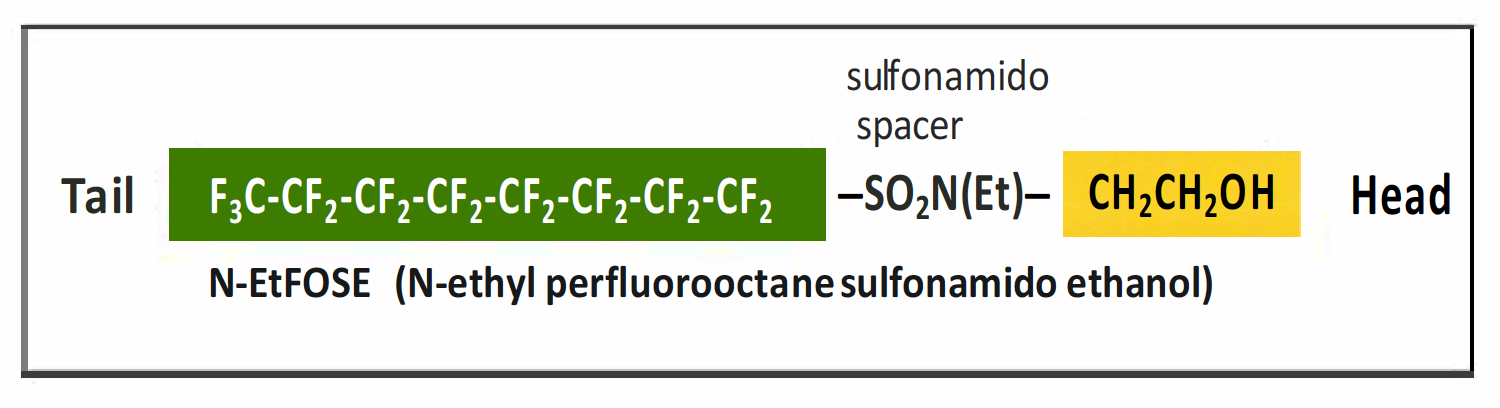
Figure 2-13. Example of a perfluoroalkane sulfonamido ethanol (FASE).
2.2.4.3 Other Polyfluoroalkyl Substances
Other polyfluoroalkyl substances shown in Figure 2-5 include:
- polyfluoroalkyl ether acids, including polyfluoroalkyl ether sulfonic acids (PFESAs) and polyfluoroalkyl ether carboxylic acids (PFECAs)
- chloropolyfluoroalkyl ether acids
- chloropolyfluoroalkyl acids
As discussed in Section 2.4.6, some PFAS have been developed or used as replacements for other PFAS that are phased out of use and production.
One replacement compound for the use of PFOA as a polymerization aid in the production of PTFE is a polyfluoroalkyl ether carboxylic acid: ammonium 4,8-dioxa-3H-perfluorononanoate (CF3OCF2CF2CF2-OCHFCF2COO–NH4+ (CAS No. 958445-44-8), commonly referred to by the trade name ADONA (Gordon 2011). The chemical structure is shown in Figure 2-14.
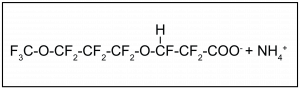
Figure 2-14. Chemical structure for ADONA ammonium salt.
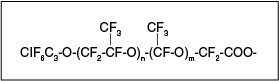
Other replacement polymerization compounds for the manufacture of PTFE and polyvinylidene fluoride (PVDF) include polyfluoroalkyl ether acids, also referred to as cyclic or polymeric functionalized perfluoropolyethers (PFPEs) (Wang et al. 2013). A sample chemical structure of a chloropolyfluoroalkyl ether acid is shown in Figure 2-15.
Figure 2-15. Sample chemical structure for a chloropolyfluoroalkyl ether acid.
2.2.5 Chemical Manufacturing
To differentiate among PFAS in understanding a conceptual site model for environmental risk assessment, it is important to know about the chemical manufacturing processes. The various manufacturing processes produce different types of PFAS, such as linear and branched isomers (as discussed in this section), which may affect the environmental fate, treatment, toxicology, and site forensics for these chemicals. The type of PFAS that might be formed by the transformation of precursor PFAS at or related to an environmental release site also may depend on the manufacturing process (refer to the family tree in Figure 2-5).
2.2.5.1 Processes
Two major processes, electrochemical fluorination (ECF) and fluorotelomerization, have been (and are) used to manufacture PFAS that contain perfluoroalkyl chains: side-chain fluorinated polymers, PFAAs and polyfluoroalkyl surfactants (USEPA 2003; Benskin, DeSilva, and Martin 2010; KEMI 2015; OECD 2018). The fluorotelomerization process may also be characterized as “oligomerization,” as it involves, for example, using tetrafluoroethylene (TFE) monomer and adding one to nine TFE monomers to form a perfluoroalkyl chain (Kissa 2001; Rao and Baker 1994). ECF and fluorotelomerization can be used to create some of the same PFAS, as shown on Figure 2-4. PFSAs are produced only using the ECF process, whereas PFCAs can be produced by both ECF and fluorotelomerization (USEPA 2003; CONCAWE 2016).
More than 600 intermediate processes have been used to further produce certain PFAS and the associated final products. Further discussion of the intermediate processes may be found in the general scientific literature and numerous textbooks specifically written about fluorinated organics and fluoropolymers (Banks, Smart, and Tatlow 1994).
Electrochemical Fluorination (ECF)
The Simons ECF process was licensed by 3M in 1945; 3M subsequently built an ECF pilot in 1949 and started commercial production in 1951 (3M Company 1999) In the ECF process, an electric current is passed through a solution of an organic feedstock and liquid anhydrous hydrogen fluoride, which causes the hydrogen atoms to be replaced by fluorine atoms, thereby creating carbon-fluorine bonds (3M Company 1999; USEPA 2003; Buck et al. 2011). ECF is used to create perfluoroalkane sulfonyl fluorides (PASFs), which are the building blocks for other sulfonyl-based PFAS, as well as perfluoroalkyl carboxylate derivatives. These ECF-synthesized PFAS can contain a variable mixture of linear and branched perfluorinated isomers, as well as other homologues, byproducts, and impurities (USEPA 2003; Buck et al. 2011). The variable composition is caused by the process conditions, raw materials, and equipment used by the ECF process (3M Company 1999; CONCAWE 2016). Subsequent processes (for example, hydrolysis, base neutralization) are then used to refine the compounds (USEPA 2003).
Historically, the ECF process was primarily used to produce POSF which was then used to make PFOS-based materials. PFOS is often a terminal transformation product of POSF-based compounds. ECF was also used to produce perfluorooctane carbonyl fluoride which was then used to produce PFOA and other derivatives (for example, using perfluorooctane carbonyl fluoride to produce PFOA and its salts, such as APFO). As part of the phaseout of production of select long-chain PFAS in the United States, 3M has ceased using ECF to make certain long-chain PFAS, such as POSF-based compounds (PFOS and PFHxS) and PFOA (Buck et al. 2011; Section 2.4.1). 3M’s phaseout did not include other, shorter chain PFAS-based products, such as those based on PBSF (3M Company 2018).
Fluorotelomerization
A typical fluorotelomerization process involves the reaction of perfluoroethyl iodide (PFEI, CF3CF2-I) with tetrafluoroethylene (TFE, CF2=CF2) to yield a mixture of even-numbered carbon linear perfluoroalkyl iodides (CnF2n+1-I, n= 4, 6, 8, 10, etc.), commonly known as “Telomer A.” Telomer A is then reacted with ethylene to make “Telomer B” (fluorotelomer iodide, CnF2n+1CH2CH2-I, n= 4, 6, 8, 10, etc.). Telomer B is reacted to make fluorotelomer alcohols (FTOHs, CnF2n+1CH2CH2-OH, n= 4, 6, 8,10, etc.) Telomer A, Telomer B, and FTOHs are the basic raw materials used to manufacture fluorotelomer-based surfactant (nonpolymer) and polymer products (Kissa 2001; Rao and Baker 1994).
As part of the USEPA 2010/2015 Stewardship Program (USEPA 2018; Section 2.4.3), eight major global fluoropolymer and fluorotelomer manufacturers phased out production of long-chain fluorotelomer-based products that were potential precursors to PFOA and other long-chain perfluoroalkyl carboxylic acids (PFCAs). Today, the major global fluorotelomer manufacturers are reported to have refined their processes and predominantly manufacture short-chain (C6) fluorotelomer-based products (American Chemistry Council 2021). Some manufacturers outside of the United States (for example, China, India) have not phased out long-chain PFAS production (Song et al. 2018).
Fluorotelomerization has been primarily used to produce linear (straight-chain) PFAS isomers with an even number of carbon atoms (Buck et al. 2011), although some sources indicate that the process can also produce compounds with an odd number of carbons and branched chains (Lindstrom, Strynar, and Libelo 2011; Danish EPA 2015).
2.2.5.2 Linear and Branched Isomers of PFAS
Many PFAS may be present as mixtures of linear and branched isomers (Figure 2-16) depending on the manufacturing process that was used. These structural differences are important because they may affect how the compounds behave in the environment and may provide an indicator of their source. Structural differences are described below:
- A linear isomer is composed of carbon atoms bonded to only one or two carbons, which form a straight carbon backbone. There can be only one linear isomer in a Cn homologue (compounds with the same number of carbons in their tail) series.
- In a branched isomer, at least one carbon atom is bonded to more than two carbon atoms, which forms a branching of the carbon backbone. There can be many isomers per Cn homologue series.
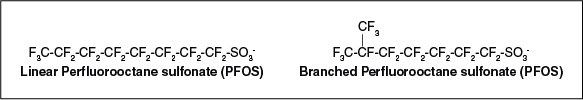
Figure 2-16. Linear and one branched isomer of PFOS.
For simplicity, both linear and branched isomers are abbreviated using the same acronym in this document. Note that other nomenclature conventions further identify PFAS by labeling linear isomers (for example, n-PFOS) and branched isomers based on the location of the branch in the carbon chain (for example, 5m-PFOS) (Benskin, DeSilva, and Martin 2010).
The formula “CnF2n+1-” (where n is greater than or equal to 3) includes linear and branched structures. For example, PFOS and PFHxS are routinely present in environmental samples as a mixture of linear and branched isomers (Beesoon et al. 2011; Beesoon et al. 2012; Benskin, DeSilva, and Martin 2010).
Accurate quantification of PFAS that are mixtures of linear isomers and branched isomers in environmental matrices can be difficult (Riddell et al. 2009). However, the relative contributions of isomers may be useful in understanding sources of PFAS and the age of the source, because the production of isomers varies by manufacturing processes. For example, as discussed above, the fluorotelomerization process has been primarily used to produce mostly linear PFAAs, whereas the ECF process produces a mixture of linear and branched PFAA isomers (Table 2-4). Refer to Section 10.5 for more information on PFAS source identification. The presence of linear and branched isomers may also have implications for partitioning, transport, and bioaccumulation (Section 10.5.1.1).
Table 2-4. Manufacturing processes and potential PFAAs produced
| Manufacturing Process | Commonly Found Polyfluorinated Substance (Precursors) | Potential PFAAs Produced |
|---|---|---|
| Fluorotelomerization | FTS1 | Linear PFCAs3 |
| FTCA2 | Linear PFCAs3 | |
| FTOH | Linear PFCAs3 | |
| Electrochemical fluorination | FASE | Branched and linear PFCAs Branched and linear PFSAs |
| FASAA | Branched and linear PFCAs Branched and linear PFSAs |
|
| 1Fluorotelomer sulfonic acid: for example, may be found at AFFF sites 2Fluorotelomer carboxylic acids: for example, 5:3 FTCA may be found in landfill leachate 3Under certain instances, can produce mixture of linear and branched PFCAs |
||
Updated September 2023.