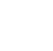7 Human and Ecological Health Effects of select PFAS
The PFAS Team developed a Human and Ecological Effects training video with content related to this section.
This section discusses both the information related to assessing health effects of PFAS in humans (Section 7.1) and the adverse effects on ecological (nonhuman) species (Section 7.2). This is an active area of scientific research. Section 7.1 provides information on human biomonitoring and exposure, toxicokinetics, toxicology in mammalian species, and human epidemiology for long-chain and short-chain PFAAs and the per- and polyfluorinated ether carboxylates (PFECAs) commonly known as the GenX chemicals and ADONA. The section is supplemented by additional material on each of these topics, which is included as Section 17.2. Section 7.2 is organized to include ecological toxicology information on invertebrates (aquatic, benthic, terrestrial), vertebrates (fish, birds, reptiles, amphibians, mammals), and plants.
For further information on the scientific names and carbon chain length of PFAAs addressed in these sections, see Section 2.2 of this document. Use of the human health effects information in guidance values is discussed in Section 8.3 and in site risk assessment in Section 9.1.
| Section Number | Topic |
| 7.1 | Human Health Effects |
| 7.2 | Ecological Toxicology |
7.1 Human Health Effects
The PFAS discussed in this section and in Section 17.2 include perfluorocarboxylic acids (PFCAs) with 4–14 carbons and perfluorosulfonic acids (PFSAs) with four or more carbons. Also covered are several PFECAs that are used as replacements for PFOA as processing aids in production of certain fluoropolymers, including the GenX chemicals, hexafluoropropylene oxide dimer acid (HFPO-DA) and its ammonium salt, (also known as perfluoro-2-propoxypropanoic acid [PFPrOPrA] and ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoate, respectively) and 4,8-dioxa-3H-perfluorononanoate, commonly known as ADONA. In addition, Section 17.2.6 reviews other ether and polyether PFAS, fluorotelomer alcohols, and fluorotelomer sulfonic acids. They are included because they are of current interest and health effects data are available. There is little or no publicly available health effects information for most of the many other PFAS used in commerce (Section 2.5), including precursors that can be converted to PFAAs in the environment and in the human body.
The best studied PFAAs are PFOS and PFOA, although considerable information is available for some other PFAS, including PFNA, PFHxS, PFBA, PFBS, and the GenX chemical HFPO-DA. Laboratory animal toxicology studies and human epidemiological studies suggest health effects that may occur as a result of long-term exposure to PFOA and PFOS at environmentally relevant levels. Figure 7-1 summarizes current health effects information, the references for which are discussed in this section. The other PFAS mentioned above cause generally similar effects in animal studies, with toxicity generally occurring at higher doses for the short-chain PFAAs than for long-chain PFAAs. These health effects, discussed in more detail in Sections 7.1.3, and 7.1.4 are the basis for current guidance values and regulations for PFOA, PFOS, and several other PFAS. These are available in the Water and Soil Regulatory and Guidance Values Table Excel file.
Figure 7-1. Some health effects of PFOA and/or PFOS identified from published studies (not exhaustive).
USEPA has finalized its toxicity assessments for the following PFAS:
- the GenX chemicals (USEPA 2021)
- PFBS (USEPA 2021)
- PFBA (USEPA 2022)
- Perfluoropropanoic acid (PFPrA)(USEPA 2023)
- Lithium bis[(trifluoromethyl)sulfonyl]azanide (HQ-115)(USEPA 2023)
- PFHxA (USEPA 2023)
As of August 2023, draft IRIS assessments were available for the following PFAS:
- PFHxS (USEPA 2023)
- PFDA (USEPA 2023)
The IRIS assessment of PFNA is under development.
USEPA (USEPA 2021; 2021) developed draft updated toxicity assessment for PFOA and PFOS which were used as the basis for the updated PFOA and PFOS interim drinking water Health Advisories (USEPA 2022; 2022). The USEPA (2021) also made a final regulatory determination to establish drinking water standards (maximum contaminant levels, MCLs) for PFOA and PFOS and proposed these standards in 2023 (USEPA 2023). As part of this effort, the draft updates of the USEPA toxicity evaluations for PFOA (USEPA 2021) and PFOS (USEPA 2021) were reviewed by the USEPA Science Advisory Board (SAB) (USEPA Science Advisory Board 2022). In response to input from the SAB, the USEPA (2021)) draft toxicity assessment for PFOA and PFOS was revised (USEPA 2023; 2023), and the revised draft assessment were used as the basis of the proposed USEPA (2023) MCLs for PFOA and PFOS.
Much of the information presented here is recent, and new studies continue to become available. Additionally, it should be noted that it was not possible to include all relevant citations, particularly for those compounds with large health effects data sets. Further information on the topics in this section can be found in databases such as the National Library of Medicine’s PubMed (a database containing citations to relevant peer-reviewed publications), and in reviews such as Kirk et al. (2018) and Lau (2012), and in several chapters of the Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profile (ATDSR 2021); DeWitt (2015), and NICNAS (2018) for PFAS in general. Some references for specific PFAS are included in this list:
- PFOA: Australia Government DOH (2018); USEPA (2016); USEPA (2016); NJDWQI (2017) NJDWQI (2017); USEPA (2023)
- PFOS: USEPA (2016); USEPA (2016); MDH (2019); NJDWQI (2018); USEPA (2023)
- PFNA: NJDWQI (2015)
- PFBS: MDH (2022); USEPA (2021)
- PFBA: USEPA (2021); MDH (2018)
- PFHxS: MDH (2020)
- PFHxA: MDH(2021) and USEPA (2022)
- GenX chemicals: RIVM (2016), Chemours (posted online by NC DEQ (2018)); and USEPA (2021)
- Short-chain PFAAs: Buck (2015) and Danish EPA (2015)
- PFECAs: Buck (2015); NJDEP (2021)
Human biomonitoring and sources of exposure are addressed in Section 7.1.1. Information on serum levels of long-chain PFAAs from communities with contaminated drinking water is presented in Table 17-6. The unique toxicokinetic properties of PFAS are discussed in Section 7.1.2. Table 17-7 summarizes available data on PFAS elimination half-lives in humans and experimental animals. The numerous reviews of potential epidemiological associations of health endpoints with PFAAs are discussed in Section 7.1.3. Toxicology studies in mammalian species are summarized in Section 7.1.4, and more detailed toxicology information is presented in Section 17.2.5 and Table 17-8 Toxicological Effects Excel file (last updated November 2021). Section 7.1.5 discusses PFAS mixtures. Section 7.1.6 includes information about using new approach methodologies for evaluating PFAS. Section 7.1.7 provides information about regulating PFAS as a class. Section 7.1.8 includes information about PFAS inhalation toxicity, and Section 7.1.9 includes data gaps and research needs.
7.1.1 Human Biomonitoring and Sources of Exposure
Numerous human biomonitoring studies (CDC 2022; Olsen et al. 2017) have demonstrated that certain PFAS, particularly long-chain PFAAs, are present in the blood serum of most U.S. residents. Long-chain PFAAs, with half-lives of one to several years, are slowly excreted in humans. Therefore, serum levels are indicators of long-term exposure to long-chain PFAAs and do not fluctuate greatly with short-term variations in exposure. Serum PFAA concentrations originate from direct exposure to the compounds and from metabolism of precursor compounds to PFAAs within the body (reviewed in Kudo (2015)). The largest U.S. general population biomonitoring studies are from the National Health and Nutrition Examination Survey (NHANES), a nationally representative survey conducted by the Centers for Disease Control and Prevention (CDC), which began monitoring for PFAS in 1999–2000 (Figure 7-2). As can be seen in Figure 7-2, serum PFAS levels in the general population have declined over time, most notably for PFOS. The most recent NHANES monitoring data (2017–2018) includes eight PFAAs (PFOA, PFOS, PFNA, PFHxS, PFHxA, PFDA, PFUnDA, PFHpS) and four other PFAS (GenX, ADONA, 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid, MeFOSAA); five additional PFAS (PFBS, PFHpA, PFDoDA, PFOSA, EtFOSAA) that were infrequently detected in earlier rounds of NHANES were not monitored in 2017–2018 (CDC 2022). Other adult U.S. general population biomonitoring data come from four studies of blood donors in 2000–2015 (Olsen et al. 2017) and the California Environmental Contaminant Biomonitoring Program (CA OEHHA 2011).
Figure 7-2. Geometric mean serum concentrations (ng/ml) of selected PFAAs (NHANES, 1999-2016).
In the general population, where this is no specific source of PFAS contamination and PFAA concentrations in drinking water and serum are in the typical “background” range, the primary sources of exposure to PFAAs and their precursors appear to be food and food packaging, and consumer products (particularly nonpolymer aftermarket treatments and coatings; Section 2.5), and house dust formed from such consumer products (Trudel et al. 2008; Fromme et al. 2009; Vestergren and Cousins 2009; Beesoon et al. 2012; Gebbink, Berger, and Cousins 2015). PFAS have been detected in air (ATSDR 2021), and inhalation is therefore an additional potential exposure pathway. Serum levels of PFOS and PFOA documented by NHANES data appear to indicate that the phaseout of production and use of these chemicals in most products has resulted in decreased PFOS and PFOA exposures for the general population from these sources. As this occurs, the relative contribution from drinking water to these PFAAs will increase (where they are present in the drinking water).
In communities near sources of PFAS contamination, exposures that are higher than those in the general population can result from ingestion of contaminated drinking water or consumption of fish from contaminated waters. As PFAS concentrations in drinking water increase, the contribution of drinking water to the total body burden increases and typically dominates an individual’s exposure. Information on serum levels of long-chain PFAAs from communities with contaminated drinking water in several U.S. states and other nations is found in Table 17-6. Finally, occupational exposures to workers (for example, in fluoropolymer manufacturing facilities) can be higher than exposures from environmental media.
Specific considerations and exposure routes relevant to PFAS exposures in the fetus, breast-fed and formula-fed infants, and young children are discussed in Section 17.2. Also see Section 17.2.2 for additional discussion of human biomonitoring and sources of human exposure.
7.1.2 Toxicokinetics
Toxicokinetics refers to the absorption, distribution, metabolism, and excretion of toxic substances. PFAAs and some other types of PFAS discussed herein have unique toxicokinetic properties as compared to other types of persistent organic pollutants (POPs). Unlike most other bioaccumulative organic compounds (for example, dioxins, PCBs), PFAAs do not have a high affinity for adipose tissue (fat). In contrast, PFAAs are water soluble, have an affinity for proteins, and generally distribute primarily to the liver and blood serum (Bischel et al. 2011; Lau 2012, 2015; Kato, Ye, and Calafat 2015). Some PFAAs have also been found in kidney, bone, lung, brain, and other organs in laboratory animals and/or limited human studies (Bogdanska et al. 2011; Perez et al. 2013; reviewed in USEPA 2023). PFAAs, GenX chemicals, and ADONA are not metabolized (meaning they do not break down to other PFAS). However, some PFAS that are PFAA precursors (for example, fluorotelomer alcohols, Section 17.2.5.2) can be metabolized to PFAAs within the body.
Table 17-7 summarizes available data on PFAS elimination half-lives (the half-life is the length of time it takes for half of the chemical to be eliminated from the body) in humans and experimental animals. In general, short-chain PFAS are excreted more rapidly than longer chain PFAS in humans and other mammalian species. As discussed in more detail in Section 17.2.3, the excretion rates (for example, elimination half-lives) for specific PFAS can vary substantially between species, and in some cases between males and females of the same species. Half-lives in laboratory animals (rodents and nonhuman primates) generally range from hours to several months for long-chain PFAS, and hours to several days for short-chain PFAS. Human half-lives for PFAS are longer than in other mammalian species, with estimates of several years for long-chain PFAAs and several days to one month for shorter chain PFAAs such as PFBA, PFHxA, and PFBS. Because of the much longer human half-lives, animal-to-human comparisons must account for the much higher internal dose (for example, blood serum level) in humans than in animals from the same administered dose.
Toxicokinetics relevant to developmental exposures to PFAAs are important because developmental effects are considered to be sensitive endpoints for toxicity of long-chain PFAAs, and some human studies have found associations of long-chain PFAAs with decreased fetal growth. PFAAs cross the placenta (reviewed in Lau (2012) and Kudo (2015)) and are present in breast milk (Luebker et al. 2005; White et al. 2009; Kato, Ye, and Calafat 2015), and long-chain PFAAs have been found in cord blood, for example, (Wang et al. 2019), and amniotic fluid (Stein et al. 2012; Zhang et al. 2013). In human infants, exposures from breast milk result in substantial increases in long-chain PFAA serum levels during the first months after birth (Fromme et al. 2010; Mogensen et al. 2015). Due to the higher rate of fluid consumption by infants versus older individuals (USEPA 2019), exposures to infants from formula prepared with PFAS-contaminated water are also higher.
Toxicokinetic factors called clearance factors represent the volume of blood from which a substance is removed per unit time (L/kg/day). Clearance factors have been used to relate external doses (ng/kg/day) of PFOA and PFOS to steady-state serum levels (ng/L). When combined with average water ingestion rates (USEPA 2023), these clearance factors have been used to predict that the expected average increases in the levels of PFAS in blood serum from long-term drinking water exposure are at least 100-fold higher than the concentration in the drinking water (Bartell 2017; NJDWQI 2017; Post, Gleason, and Cooper 2017). See Section 17.2.3.2 for more detail. Bartell (2017), Lu and Bartell (2020), and ATSDR (2022) have developed online calculators that provide estimates of an individual’s serum concentrations of PFOA, PFOS, PFNA, and PFHxS from the information that is entered, including drinking water levels of these PFAS and other relevant factors. It should be noted that these estimates are based on long-term exposure to a constant drinking water concentration and that serum PFAS concentrations are impacted by interindividual variability in both toxicokinetic factors (for example, PFAS half-lives) and the daily drinking water ingestion rate.
Finally, toxicokinetics in rodents (Loveless et al. 2006; De Silva et al. 2009) and humans (Zhang et al. 2013; Gao et al. 2015; Beesoon et al. 2011) may differ among isomers of the same PFAA.
See Section 17.2.3 for additional discussion of PFAS excretion and excretion rates, toxicokinetics relative to developmental exposure, the relationship of human exposure to serum levels, and isomer-specific toxicokinetics.
7.1.3 Human Epidemiology Studies
The epidemiological database for long-chain PFAAs, particularly PFOA and PFOS, is more extensive than for many other environmental contaminants. Many of the studies are recent, and the number of available studies is continually increasing. USEPA (2023) identified over 400 human epidemiology studies of PFOA and/or PFOS from searches of the following databases through February, 2022: Pub Med (National Library of Medicine); Web of Science (Thomson Reuters); ToxLine (incorporated into PubMed post 2019); and TSCATS (Toxic Substances Control Act Test Submissions). Many of these studies also evaluated other long-chain PFAAs and/or other PFAS. Some effects, such as changes in serum lipids, liver biomarkers, uric acid levels, thyroid endpoints, vaccine response, and fetal growth, have been evaluated in multiple studies and populations, while only one or a few studies were located for some other effects
These studies can be categorized based on the type of population evaluated: general population, communities with contaminated drinking water, or occupationally exposed workers. Almost all of these studies were published after 2009, with the exception of a small number of occupational studies from a few years prior to that time.
Although discussion of individual epidemiological studies is beyond the scope of this section and the corresponding appendix section, evidence for associations and/or causality for some PFAAs and certain health effects (for example, increased cholesterol, increased liver enzymes, decreased vaccine response, thyroid disease, and for PFOA, some types of cancer) has been evaluated by various academic researchers and government agencies. USEPA (2023; 2023) has concluded that the noncancer human health effects with the strongest evidence for association with PFOA and PFOS are increased serum cholesterol, increased serum alanine aminotransferase (ALT; a marker of liver damage), decreased vaccine response, and decreased birth weight. USEPA (2023) has also concluded that PFOA is linked to testicular and kidney cancer in humans. The conclusions of some of these evaluations are discussed briefly below, with additional detail provided in Section 17.2.4.
For some health endpoints, including increased serum cholesterol, there is general consensus for consistent evidence for association with one or more long-chain PFAAs. Conclusions differ among evaluations by different groups of scientists for other endpoints, noting that the earlier evaluations considered fewer studies than the more recent evaluations. For additional endpoints, data are too limited to make a conclusion, results are inconsistent, or there is no evidence for an association. The general reviews cited in Section 17.2.4 include detailed discussions of epidemiological data for PFOA, PFOS, and PFNA.
As shown in Figure 7-1, associations in human epidemiological studies of PFAAs (primarily PFOA and PFOS) for some endpoints (for example, increased liver enzymes, decreased fetal growth, decreased vaccine response) are consistent with animal toxicology studies (Section 7.1.4). For serum lipids (for example, cholesterol), observations of decreased cholesterol in rodents in some studies, while cholesterol is increased in humans, may result from the higher fat content in the diets of humans as compared to the diet used in most laboratory animal studies and/or large differences in the exposure levels in human versus animal studies (Tan et al. 2013; Rebholz et al. 2016 ; NJDWQI 2017; USEPA 2023).
Associations of some health endpoints with certain PFAAs are generally, although not totally, consistent, and some evaluations have concluded that the data for certain effects support multiple criteria for causality. Historically, risk-based toxicity factors (reference doses for noncancer effects and slope factors for cancer risk) developed by most government agencies are based on dose-response relationships from animal data, with the human data used to support the hazard identification component of toxicity factor development. One factor that has precluded the use of human data in the dose-response component of toxicity factor development is the concurrent exposure to multiple PFAAs in most or all study populations. Because serum levels of co-occurring PFAAs tend to correlate with each other, special modeling approaches must be used to determine the dose-response relationship for individual PFAAs, and the use of these approaches is currently increasing. That fact notwithstanding, Hölzer, Lilienthal, and Schümann (2021) and Schümann, Lilienthal, and Hölzer (2021) developed Human Biomonitoring Values (serum levels below which adverse effects are not expected) and the European Food Safety Authority (EFSA 2020) developed a Tolerable Weekly Intakes (TWI) of 4.4 ng/kg body weight for the sum of four PFAS (PFOA, PFOS, PFNA, and PFHxS) in food based on human data for decreased vaccine response from the general population. These values are lower than many of the values that are based on toxicity data from animals. More recently, California EPA and USEPA have developed draft reference doses for PFOA and PFOS (CA OEHHA 2023; USEPA 2023; 2023), as well as for PFHxS (USEPA 2023) and PFDA (USEPA 2023), and a draft cancer slope factor for PFOA (USEPA 2023; CA OEHHA 2023) based on human general population data that are far below current values based on animal data.
The National Academies of Science, Engineering, and Medicine (NASEM 2022), at the request of ATSDR and the National Institute of Environmental Health Sciences (NIEHS), developed recommendations for testing for PFAS exposure and clinical monitoring for exposed individuals. ATSDR will consider these recommendations in updating its guidance to clinicians regarding PFAS. As part of its work, NASEM (2022) developed “strength of evidence” determinations based on human data for a variety of health effects that are collectively applicable to the total serum concentration of the seven PFAS (PFOA, PFOS, PFHxS, PFNA, PFDA, PFUnDA, and methyl-perfluorooctane sulfonamide [MeFOSA]) currently included in the CDC’s National Report on Human Exposure to Environmental Chemicals (CDC 2022). The NASEM relied on risk-based assessments conducted by EFSA (2020) and the German Human Biomonitoring Commission (2016), (2018) to identify PFAS levels in serum or plasma to inform clinical care. The NASEM determined that: (1) adverse health effects related to PFAS exposure are not expected at PFAS serum levels less than 2 ng/mL; (2) there is a potential for adverse effects, especially in sensitive populations, for PFAS serum levels between 2 and 20 ng/mL; and (3) there is an increased risk of adverse effects for PFAS serum levels above 20 ng/ml. The NASEM recommended PFAS exposure reduction—if a source has been identified—for the two highest categories of PFAS serum concentrations. NASEM (2022) further recommended that clinicians should “offer PFAS [blood] testing to patients likely to have a history of elevated exposure,” including those with potential occupational exposure and those who have lived in communities with known or potential PFAS contamination. The NASEM also recommended that clinicians conduct health screening for several health conditions when the sum of the seven PFAS in serum exceeds the recommended benchmarks. Also see the discussion of the NASEM document in Section 7.1.8, Regulation of PFAS as a Class.
See Section 17.2.4 for additional discussion of epidemiologic studies that have been conducted on PFAS.
7.1.4 Animal Toxicology Studies
This section focuses on the most notable toxicological effects in mammalian studies of certain PFCAs, PFSAs, and PFECAs. All PFAS covered in this section for which data are available cause increased liver weight; additional effects common to some of these PFAS include immune system, hematological (blood cell), and developmental toxicity, as well as more severe types of liver toxicity. Of the four PFAS that have been tested for carcinogenicity in rodents, PFOA, PFOS, and the GenX chemical HFPO-DA caused tumors while PFHxA did not.
In general, toxicity is dependent on both intrinsic potency of the compound (Gomis et al. 2018) and its toxicokinetics. Longer chain PFAAs are generally toxic at lower administered doses than shorter chain compounds because their slower excretion results in a higher internal dose from the same administered dose. Similarly, for those PFAS that are excreted much more rapidly in female rats than in males (Section 7.1.2 and Table 17-7), higher doses in females than in males are needed to achieve the same internal dose.
Toxicological data from animal studies are used as the basis for many human health toxicity factors (for example, reference doses, cancer slope factors) for PFAS. However, as noted above, both California and the USEPA have based recent draft reference doses and/or cancer slope factors for on human data. Certain European toxicity factors are also based on human data (Section 7.1.3) (also see Sections 8.3 and 9.1). Unlike most other environmental contaminants, PFAS have been associated with health effects in humans at much lower exposure levels than the doses used in animal toxicology studies.
Table 17-8 Toxicological Effects Excel file provides information on toxicological effects in mammalian species (hazard identification information) for the following PFAS:
- PFCAs including PFBA, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, and PFDoA
- PFSAs including PFBS, PFPeS, PFHxS, PFHpS, and PFOS
- PFECAs including ADONA and the GenX chemical HFPO-DA.
Section 17.2.5.1 also summarizes information on systemic effects; reproductive and developmental effects, and chronic toxicity and tumorigenicity of these PFAS.
- Largest publicly available toxicological data sets for: PFOA and PFOS
- Considerable data for: PFBA, PFHxA, PFNA, PFDA, PFBS, and the GenX chemicals HFPO-DA and its ammonium salt
- One or a few studies for: PFHpA, PFUnA, PFDoA, PFHxS, and ADONA
- No toxicological data were located for PFPeA, PFTrDA, PFTeDA, PFPeS, PFHpS, PFNS, or PFDS.
Most toxicological studies of PFAS have been conducted in rats and mice, with a few studies in nonhuman primates (monkeys) and other species such as rabbits.
The National Toxicology Program (NTP 2019; NTP 2019) has conducted 28-day studies of seven PFAS (PFHxA, PFOA, PFNA, PFDA, PFBS, PFHxS, PFOS) in male and female rats that evaluated numerous toxicological endpoints and provided serum PFAA data for each dosed group; results of these studies are included in Table 17-8 Toxicological Effects Excel file. Although the doses at which effects occurred are not provided in this section or in the supporting appendix material, it is emphasized that No Observed Adverse Effect Level (NOAELs) and Lowest Observed Adverse Effect Level (LOAELs) vary widely between compounds for a given endpoint, between different endpoints for the same compound, and between species (and sexes in some cases) for the same compound and endpoint. Furthermore, the effects noted may not have been observed in all studies in which they were evaluated.
NTP (2020) has also conducted a chronic carcinogenicity study of PFOA administered in feed to rats that assessed the contribution of combined gestational and lactational (perinatal) exposure as compared to exposure beginning after weaning. It was concluded that there was clear evidence of carcinogenic activity in male rats based on the increased incidence of liver tumors and pancreatic acinar cell tumors, and some evidence of carcinogenic activity in female rats based on increased incidence of pancreatic acinar cell tumors. Non-neoplastic lesions were increased in the liver and pancreas in males and in the liver, kidney, forestomach and thyroid in females. There were very few significant differences in effects when exposure began in the perinatal period compared to when it began after weaning.
See Section 17.2.5.1 for additional discussion of studies in animals that have evaluated the effects of these PFAS on noncancer and cancer endpoints.
There is increasing awareness and interest in potential human exposure to PFAS other than the PFAAs and the PFECAs, GenX and ADONA, that are discussed in Section 17.2.5.1. These include ether and polyether PFAS, dicarboxylic acid polyether PFAS, fluorotelomer alcohols, and fluorotelomer sulfonic acids. Information relevant to health effects of these additional PFAS is discussed in Section 17.2.6.1.
7.1.5 PFAS Mixtures
This section provides a summary of current toxicity-based approaches for addressing mixtures of PFAS and currently available information on toxicity of PFAS mixtures. This is an active area of research. Additional information is presented in Section 17.2.
Although humans are generally exposed to mixtures of PFAS, relatively few studies of the toxicity of PFAS mixtures, including defined mixtures (i.e., mixtures for which the identities and concentrations of the components are known) or complex mixtures (i.e., mixtures for which the identities and concentrations of the components are not fully characterized) such as AFFF, were located; they are summarized in Section 17.2.7.2. These include in vitro studies of nuclear receptor activation in cultured cells transfected with the receptor of interest, and toxicity in cultured cells; in vivo studies in zebrafish (a model species for human toxicity) and mammalian species; and epidemiologic studies of a population exposed to AFFF in drinking water.
7.1.5.1 Approaches for Assessing Toxicity of PFAS Mixtures
Multiple PFAS are present in the blood serum of almost all U.S. residents (Section 7.1.1), and PFAS often occur as mixtures of individual chemicals in the environment (for example, drinking water, fish, soil, and air). Therefore, approaches to assess the toxicity of PFAS mixtures are needed. Development of approaches for consideration of the health effects of mixtures of PFAS is a priority for USEPA, ATSDR, and other U.S. federal agencies, as discussed at a recent National Academy of Sciences (NAS) workshop on federal research on human health effects of PFAS (NAS 2021), and USEPA (2023) developed a draft framework for evaluation of non-cancer risk of PFAS mixtures that was reviewed by the USEPA Science Advisory Board (2022). Approaches to assessing the toxicity of PFAS mixtures have also been the subject of several peer-reviewed publications (discussed below), including Peters and Gonzalez (2011), Bil et al. (2021), Cousins et al. (2020), and Goodrum et al. (2021). However, as discussed at the NAS workshop, relevant data on several aspects of this topic are limited. For example, there is a lack of information on in vivo and in vitro toxicity of PFAS mixtures, as well as on health effects in human populations when exposure to multiple PFAS has occurred (NAS 2021).
As is the case for mixtures of environmental contaminants in general, the toxicity of PFAS mixtures can be evaluated through studies of defined or undefined mixtures (mixtures of known concentrations of individual PFAS or complex mixtures that may include both known and unidentified PFAS, respectively). The small database of currently available toxicity studies of PFAS mixtures is summarized in Section 17.2. These include a few studies of the undefined PFAS mixtures found in AFFF and a limited number of studies of defined PFAS mixtures, including in vitro studies of nuclear receptor activation in transfected cultured cells, and toxicity studies in cultured cells and zebrafish. Notably, no rodent or primate studies of defined mixtures of PFAS were located. When evaluating exposure and health effects for PFAS mixtures in drinking water or other media, it must be emphasized that identification of the PFAS that are present is dependent both on the composition of the mixture and the suite of PFAS detected by the analytical method that was used (McDonough et al. 2019). Methods currently used for routine analysis are described in Section 11.2. Additional analytical methods (for example, nontarget analysis) that are primarily used in research studies can identify numerous additional PFAS not currently included in standard methods.
As discussed in USEPA and ATSDR guidance for risk assessment of mixtures (USEPA 1986; USEPA 2000; ATSDR 2018), toxicological interactions among components of a mixture may include dose additivity, response additivity, synergism, and antagonism. Dose additivity is based on the assumption that the chemicals in the mixture have the same mode of action (MOA) and cause the same effects, and differ only in potency. The components are assumed to have similarly shaped dose-response curves, and the effect caused by the mixture is assumed to be the sum of the effects caused by each of the individual chemicals present. Because MOA information for PFAS is incomplete, the draft USEPA (2023) framework for evaluation of risks of PFAS mixtures assumes dose additivity based on similarity of toxicological endpoint/health effects without requiring a common MOA. Response additivity is based on the assumption that the toxic effect of a mixture can be predicted by summing the doses of each chemical in the mixture (relative to each chemical’s critical effect dose) such that the contribution from each chemical to the overall effect of the mixture is not influenced by the other chemicals. For example, when assuming additivity, the sum of the incremental cancer risks of multiple individual co-occurring carcinogens can be used to estimate the total cancer risk of the mixture. Toxicological interactions of components of mixtures may also be synergistic (greater than additive) or antagonistic (less than additive), resulting in larger or smaller effects than would occur with dose additivity or response additivity.
As discussed in this section, currently available toxicological information for PFAS does not clearly support a specific approach for conducting risk assessments of PFAS mixtures. While the toxicological effects of many PFAS are generally similar, the most sensitive toxicological endpoints may vary among PFAS. Additionally, multiple MOAs for PFAS toxicity are likely; these MOAs appear to be complex, and currently are not well understood. Furthermore, the MOA for a specific PFAS may not be the same for all effects (for example, hepatic toxicity, developmental toxicity), and a specific effect may not occur through the same MOA for all PFAS (for example, different MOAs for liver toxicity of PFOA and PFOS; Peters and Gonzalez 2011; NAS 2021). In the limited number of studies of PFAS mixtures, additive, synergistic, and antagonistic interactions among PFAS have been observed. As noted by Wolf et al. (2014), toxicological interactions of PFAS are dependent on the identities and concentrations of the PFAS in the mixture, the biological model, and the endpoint being evaluated (see Section 17.2).
Some regulatory agencies consider the cumulative toxicity of PFAS that co-occur in environmental media, while other agencies consider PFAS individually as required by their regulatory process and/or the scientific uncertainty as to whether co-occurring PFAS are sufficiently similar to consider them as a group. Several approaches that have been proposed to address the toxicity of PFAS mixtures are discussed in Section 17.2.7.1. The goal of these approaches is to facilitate the risk assessment of PFAS mixtures detected at contaminated sites and to develop health-protective guidelines that potentially account for the combined effects of multiple PFAS. Some general approaches for assessing the cumulative effects of exposure to PFAS, as related to site risk assessment, are discussed in Section 9.1.3.1. Examples of the application of such approaches to PFAS are reviewed by Cousins et al. (2020), who also provided a “summary of existing or proposed grouping approaches based on the sum of various PFAS in drinking water.” Most of the approaches that have been proposed assume dose additivity, although scientific uncertainties are associated with this assumption, as discussed above and in Section 17.2.7.1.
7.1.5.2 Toxicology Studies of PFAS Mixtures
The relatively few studies of the toxicity of defined mixtures of PFAS that were located are summarized in Section 17.2.7.2. These include in vitro studies of nuclear receptor activation in cultured cells transfected with the receptor of interest, toxicity in cultured cells and zebrafish (a model species for human toxicity), and a few mammalian studies.
7.1.6 Evaluating PFAS using New Approach Methodologies
Traditional toxicity testing methods using in vivo mammalian models are time-, cost-, and labor-intensive. As noted in Section 17.2.8, including Table 17-8 Toxicological Effects Excel file, mammalian (most commonly rodent) toxicity information is available for only a relatively small group of PFAS, including a number of long- and short-chain PFCAs and PFSAs, certain fluorotelomer alcohols and fluorotelomer sulfonates, and several of the per- and polyfluoroether replacements, with PFOS and PFOA having the most extensive data sets. Under current USEPA risk assessment guidelines used by most states, in vivo mammalian laboratory animal or human data are required for development of chemical-specific toxicity factors (for example, reference doses) used as the basis for standards and guidance values for PFAS in drinking water and other environmental media. It is therefore important that in vivo mammalian data continue to be made available for those PFAS with a high level of public health concern, such as those detected at elevated concentrations in drinking water. However, in vivo mammalian studies are not feasible for all of the individual PFAS, of which there are thousands (USEPA 2020). Additionally, USEPA aims to refine and reduce the use of mammalian species in toxicology testing and to use non-animal testing methods when appropriate. With the goal of rapidly generating toxicity, MOA, toxicokinetic, and exposure information on PFAS and reducing the use of mammalian species in testing, the USEPA is collaborating with the National Toxicology Program (NTP) of the National Institutes of Environmental Health Sciences through the REACT (Responsive Evaluation and Assessment of Chemical Toxicity) Program. New Approach Methodologies, such as rapid toxicity assays in cultured cells and zebrafish, in silico (computational) approaches, and high throughput exposure modeling are being developed and used with the goal of generating data that will inform toxicology and risk assessment of PFAS (Patlewicz et al. 2019; USEPA 2019, 2019). More information on this effort is provided in Section 17.2.8.
7.1.7 Regulation of PFAS as a Class
Recent estimates could have the total number of individual PFAS at more than 12,000 (see Section 2 and USEPA 2020). Although not all of these PFAS are commercially important, more than 3,000 individual PFAS have been found in European commerce (OECD 2018), and approximately 4,700 PFAS are in the current global marketplace (Cousins et al. 2020). Of these, Buck et al. (2021) reported that 256 PFAS are commercially relevant on a global basis. Fewer than 20 PFAS are well-studied toxicologically (all of which are nonpolymer PFAS), and all of those that have been studied have been found to be capable of causing adverse effects in animals and/or humans (Sections 7.1, 7.2, and 17.2).
Because substantial time and resources are required to adequately characterize the chemical and physical properties and potential adverse effects of an individual chemical (for example, NTP, 2022), the conclusion that it is neither feasible nor health-protective to follow the “chemical-by-chemical” regulatory paradigm for PFAS has gained increasing acceptance. Beginning with the Helsingør Statement (Scheringer et al. 2014) and continuing with the Madrid Statement (Blum et al. 2015) and Zurich Statement (Ritscher et al. 2018), groups of scientists and others have called for the coordinated regulation of all PFAS, citing their persistence, widespread use, and frequent lack of toxicity data, among other concerns. The FluoroCouncil (Bowman 2015) took issue with the Madrid Statement (Blum et al. 2015) for failing to acknowledge the fact that “fluorotechnology is essential technology,” and for ignoring scientific information that indicates differences in persistence and toxicity between long- and short-chain PFAAs and the efforts of industry to limit environmental impacts of PFAS.
One of the various regulatory strategies proposed by Blum et al. (2015), Ritscher et al. (2018), and subsequently by Cousins et al. (2019), is to limit ongoing uses of PFAS to those PFAS deemed to be “essential.” Cousins et al. (2019) proposed that essential uses of PFAS are those “uses considered essential because they are necessary for health and safety or other highly important purposes and for which alternatives have not been established.” Examples of essential uses are certain medical devices and occupational protective clothing (Cousins et al. 2019). The European Commission (2020)[2800] developed a strategy to phase out the use of PFAS in the European Union “unless it is proven essential for society.” Similarly, while noting that “a ban on all PFAS as a group is neither practical, necessary, nor achievable,” the Royal Society of Chemistry (2021)[] supported the concept of essential use for those PFAS defined as “vital or highly desirable by wider society.” Other publications have also supported the concept of “essential use” as a regulatory strategy for PFAS (Kwiatkowski et al. 2020; Dean et al. 2020), with Gluge et al. (2022) describing the information requirements and analyses necessary to regulate PFAS under the concept of essential use.
Kwiatkowski et al. (2020) also emphasized the importance of eliminating nonessential uses of PFAS as an example of a class-based approach to PFAS regulation. Other class-based regulatory strategies cited by Kwiatkowski et al. (2020) include banning PFAS in certain product categories; prioritizing research and development funding for treatment and disposal/destruction methods that are targeted to (and effective for) PFAS as a class; and development of class-based cleanup standards so that all PFAS—not just a few—are remediated.
7.1.7.1 Grouping Strategies
The Zurich Statement (Ritscher et al. 2018) and others (Cousins et al. 2020; Anderson et al. 2022) have considered the utility of and strategies for grouping PFAS for more effective regulation, as distinct from treating all PFAS as a single class. Cousins et al. (2020) offered a number of potential bases for grouping PFAS, with the selected method depending on whether the intended action is to regulate based on intrinsic properties or to inform risk assessment. Each of the proposed grouping methods has different data requirements, advantages, and disadvantages (see Cousins et al. 2020). Examples of the grouping methods proposed by Cousins et al. (2020) are summarized in Table 7-1.
Table 7-1. Grouping approaches for PFAS (excerpted and adapted from Cousins et al. 2020, CC BY-NC 3.0).
| Individual approaches | PFAS grouped | Data requirements | |
|---|---|---|---|
| Approaches based on intrinsic properties | P-sufficient approach1 | All PFAS | None |
| According to PBT/vPvB2 | PFAS that are bioaccumulative | Bioaccumulation potential | |
| According to PMT/vPvM2 | PFAS that are mobile in water | Water solubility | |
| Polymers of low concern (PLC) | Some fluoropolymers | Polymer composition, molecular weight, other properties | |
| Approaches that inform risk assessment | Arrowhead3 | Specific PFAAs and precursors | Degradation schemes |
| Total organofluorine | Extractable or adsorbable PFAS | None | |
| Simple additive toxicity | 2-20 PFAS, currently primarily PFAAs | Toxicity | |
| Relative potency factor | Multiple PFAAs | Toxicity, potency, toxicokinetics | |
| Grouping PFAS with similar adverse effects, mode of action, toxicokinetic | Limited PFAAs | Toxicity, mode of action, toxicokinetics | |
| 1 P-sufficient = grouping based on the persistence of perfluoroalkyls and on the formation of perfluoroalkyls as stable end products of precursor polyfluoroalkyls.2 PBT = persistent, bioaccumulative, toxic; vPvB = very persistent and very bioaccumulative; PMT = persistent, mobile, and toxic; vPvM = very persistent and very mobile.
3 Arrowhead = “when a representative PFAS, usually a PFAA, is managed together with its salts and precursors.” |
|||
As described in its National PFAS Testing Strategy, the USEPA (2021) is working to understand the impacts of different categories of PFAS, in part to identify which PFAS will require testing under the Toxic Substances Control Act authority. The PFAS categorization will also support the prioritization of individual PFAS or “classes” of PFAS for additional research on human exposure or toxicity. The USEPA’s categorization of PFAS is based on similarities in structure, physical-chemical properties, and toxicological properties. The USEPA plans to use these categories for hazard assessment and to support risk-based decision making and will also develop categories of PFAS based on removal technologies. The USEPA will draw on information from both of these approaches to prioritize research.
Anderson et al. (2022) considered several options for grouping of PFAS for the purpose of protecting human health from drinking water exposure and assessing risks of PFAS mixtures. No single grouping strategy was identified that was “sufficient” for all regulatory or public health risk assessment purposes. However, the study authors generally supported the conclusions that: “Not all PFAS should be grouped together, persistence alone is not sufficient for the purposes of assessing health risk, and that the nature and definition of the subgroups can only be defined on a situation-dependent and case-by-case manner” (Anderson et al. 2022).
A recent review of the properties of fluoroplastics and fluoroelastomer—two types of fluoropolymer—concluded that based on their high molecular weight, lack of biological availability, and other physical and chemical properties, these PFAS are a distinct group of PFAS that “should not be grouped with other PFAS for hazard assessment or regulatory purposes” (Korzeniowski et al. 2022).
California’s Department of Toxic Substances (DTSC) regulates PFAS as a class by prohibiting the sale of select consumer products if the product contains any PFAS and an Alternatives Analysis has determined that there are viable replacements. Supported by specific California laws that define environmental persistence and toxicity as hazard traits warranting regulation, the DTSC considers that (1) all PFAS or their degradation, reaction, or metabolic products are environmentally persistent, and (2) “nearly all” PFAS exhibit other hazard traits as defined by California law, including toxicity (Balan et al. 2021). PFAS-containing product categories that DTSC has identified for regulation include (1) carpets and rugs with perfluoroalkyl or polyfluoroalkyl substances, (2) treatments containing perfluoroalkyl or polyfluoroalkyl substances for use on converted textiles or leathers, such as carpets, upholstery, clothing, and shoes, and (3) plant fiber–based food packaging containing perfluoroalkyl or polyfluoroalkyl substances (https://dtsc.ca.gov/scp/). In August 2022, California’s legislature banned the use of any PFAS “intentionally added” to a cosmetic product manufactured, sold, or delivered into the state of California. The text of the legislation cites the “highly toxic” and “highly persistent” “class of chemicals known as PFAS” (California Assembly 2022).
The National Academies of Science, Engineering, and Medicine (NASEM 2022) developed “strength of evidence” determinations collectively applicable to the seven PFAS currently included in the CDC’s National Report on Human Exposure to Environmental Chemicals (CDC 2022) for each health effect that they considered, while recognizing that differences exist among these PFAS. Specifically, the NASEM (2022) stated: “Most people are exposed to mixtures of PFAS such that specific effects are difficult to disentangle. Considering these issues, and recognizing that some PFAS are infrequently measured, the committee provided one strength-of-evidence determination for all PFAS for each health effect, recognizing that providing one conclusion across PFAS may not account for the distinct physical, chemical, and toxicological properties of each type of PFAS.”
The European Chemicals Agency (ECHA) proposed to restrict all per- and polyfluoroalkyl substances with a single regulation (ECHA 2023). The proposed regulation cites concerns that “all PFAS” are “very persistent” in the environment and may adversely affect the health of humans and the environment. ECHA (2023) observed that if the proposal is put into effect, it would prevent the release of around 4.4 million tons of PFAS over a 30-year period.
In February 2023, Health Canada proposed to regulate all PFAS in drinking water that can be quantified with U.S. EPA Method 533, Method 537.1, or both. The sum of total detectable PFAS will be required to be no more than 30 ng/L, based on potential health effects from exposure to this group of PFAS in drinking water (Health Canada 2023).
7.1.8 PFAS Inhalation Toxicity
This section provides an overview of available information on inhalation exposure and toxicity of PFAS. While exposure to PFAS via inhalation can potentially occur both outdoors and indoors, most of the exposure studies that were identified focused on residential and nonresidential indoor environments. A review by Savvaides et al. (2021) reported that, in the available studies, the profile of PFAS detected in indoor air varied in different indoor environments and in different seasons of the year in the same location.
In general, inhalation exposure considerations differ for ionized (negatively charged) PFAS [(for example, PFAAs such as PFOA and PFOS, and perfluoroether carboxylates such as HPFO-DA, which have low volatility and occur primarily in indoor (for example, house) dust], as compared to neutral PFAS [(for example, FTOHs, FOSAs, and FOSEs), which are much more volatile and tend to occur in the vapor phase] (Table 4-1; De Silva et al. 2021). For this reason, the negatively charged and neutral PFAS are discussed in separate sections below. For example, Shoeib et al. (2011) and Ericson Jogsten et al. (2012) reported that PFAAs and neutral PFAS were both found in residential environments. However, the concentration of neutral PFAS was higher than PFAAs in residential indoor air while the concentration of PFAAs in house dust was higher than neutral PFAS. Furthermore, Gustafsson et al. (2022) reported that the total concentration of PFAS and the distribution of specific PFAS varied in different size fractions of house dust. The highest total PFAS concentration was found in the smallest particles, which represent the respirable fraction, while PFCAs were highest in the largest and smallest particles, with lower concentrations in the intermediate-size particles. Information about media-specific occurrence studies is included in Section 6.
Some neutral PFAS are “precursors” (i.e., PFAS that can be metabolized or otherwise degraded to form terminally stable PFAAs), so that inhalation exposure to PFAAs may occur both through “direct” and “indirect” pathways. For example, Gomis et al. (2016) characterized the contribution of “direct” exposure to PFOA and “indirect” exposure to 8:2 FTOH to the total serum PFOA concentrations in ski waxers exposed to PFAS via inhalation, and Chang et al. (2017) reported the metabolism of EtFOSE to PFOS in rats exposed via inhalation.
Although this section does not focus on development of reference concentrations and unit risk factors for inhalation exposure, it is noted that ECOS (2023) provides information on inhalation toxicity factors for PFAS that have been developed by some states. In general, inhalation toxicity studies that can be used to develop toxicity factors are not available for PFAS, and these inhalation toxicity factors are based on oral toxicity data and route-to-route (oral-to-inhalation) extrapolation; a recent publication (Monnot et al. 2022) supports this approach.
7.1.8.1 Negatively Charged PFAS
As mentioned above, negatively charged PFAS such as PFAAs and perfluoroether carboxylates (for example, HPFO-DA) are not highly volatile. The primary route of occupational exposure to PFAAs is “likely [to be] inhalation of aerosols complexed with airborne dusts,” and elevated serum PFAA levels in occupationally exposed individuals indicates that absorption occurs via inhalation (ATSDR 2021). A major contributor to inhalation exposure to negatively charged PFAS in the general population is indoor dust containing PFAS that originates from consumer products (for example, treated carpets and furniture) and other potential sources; such dust can also contain neutral PFAS (Savvaides et al. 2021). Although exposure to dust can occur through direct ingestion, dust can also be inhaled and then swallowed after being trapped in mucous in the respiratory tract (USEPA 2017). Floor-stripping and waxing were also reported as sources of PFAAs in airborne particulate matter (PM2.0; Zhou et al. 2022).
Depending on the individual chemical, there are limited or no laboratory animal data on the inhalation toxicity of negatively charged PFAS, including those with a large number of oral toxicology studies in laboratory animals. For example, ATSDR and/or USEPA toxicity evaluations cite two acute, one short-term, and one developmental rat inhalation study of PFOA (ATSDR 2021; USEPA, 2016), and a single acute rat inhalation study for each of three additional PFAS: PFNA (ATSDR 2021); PFOS (USEPA 2016); and GenX (USEPA 2021). In these inhalation studies, exposure was via aerosols or dust. No additional inhalation studies for PFOA or PFOS were identified in more recent draft USEPA evaluations (USEPA 2023; 2023), and no inhalation toxicity studies were identified by ATSDR (2021) for PFBA, PFHxA, PFHpA, PFDA, PFUnDA, PFDoDA, PFBS, PFHxS, or PFOS, or by USEPA for PFBS (USEPA 2021), PFBA (USEPA 2022), or PFHxA (USEPA 2023).
In toxicokinetic studies, PFOA was detected in the blood serum of rats after inhalation exposure via aerosols (Hinderliter 2003; Hinderliter, DeLorme, and Kennedy 2006). However, little data are available on extent of absorption for inhalation vs. oral exposure, and an analysis by the Michigan Department of Environmental Quality (MI DEQ 2018) of PFOA data from Hinderliter, DeLorme, and Kennedy (2006) demonstrated that the relative extent of absorption from inhalation versus oral exposure was not constant over the concentration range in the study. Inhalation exposure to PFOA, PFOS, and PFNA caused systemic effects similar to oral exposure, including hepatic toxicity (PFOA–Kennedy et al. 1986; Staples et al. 1984; PFNA–Kinney, Chromey, and Kennedy 1989; PFOS–Rusch, Rinehart, and Bozak 1979) and decreased neonatal body weight after gestational exposure (Staples et al. 1984). Nasal and/or eye irritation, and/or respiratory effects, were also reported from acute exposure to relatively high concentrations of PFOA (Rusch 1979; Griffith and Long 1980; Kennedy et al. 1986), PFNA (Kinney, Chromey, and Kennedy 1989), PFOS (Rusch, Rinehart, and Bozak 1979), and GenX (DuPont 2009).
7.1.8.2 Neutral PFAS
Neutral PFAS such as FTOHs, FOSA, and FOSE are more volatile than negatively charged PFAS. They have been found in the vapor phase in indoor environments, including residences, classrooms and offices, outdoor gear/apparel and carpet stores, and ski waxing facilities (Shoeib et al. 2011; Morales-McDevitt 2021; Gomis et al. 2016), and inhalation can be a primary human exposure route (De Silva et al. 2021). Furthermore, Titaley et al. (2022) reported high concentrations of neutral PFAS in AFFF and predicted that vapor intrusion of these PFAS will occur from AFFF-contaminated groundwater.
The toxicokinetics of 6:2 FTOH and 8:2 FTOH in rats were reported to be similar after inhalation and oral exposure, including systemic absorption and the profile of metabolites formed (Himmelstein et al. 2012). As discussed in Section 17.2.6.2, metabolites of FTOHs include terminal PFCAs (for example, formation of PFHpA, PFOA, and PFNA from 8:2 FTOH), as well as persistent toxic metabolites in some cases (for example, 5:3 FTA from 6:2 FTOH) (Kabadi et al. 2020). Although no inhalation toxicology studies of FTOHs in laboratory animals were identified, the similarity in inhalation and oral toxicokinetics, including metabolite profiles, suggests that systemic toxicity is likely similar from both exposure routes. Similarly, although no inhalation toxicity studies of FOSA or EtFOSE were identified, FOSA was reported to be metabolized to PFOS in humans believed to be exposed through inhalation (Olsen et al. 2005) and EtFOSE was metabolized to PFOS in rats (Chang et al. 2017).
7.1.9 Data Gaps and Research Needs
Although many studies relevant to health effects of PFAAs have become available in the last few years, important data gaps remain for most of the PFAAs and PFECAs discussed here and in Section 17.2, as well as for many additional PFAS used in commerce or found in AFFF. The data gaps (discussed in more detail in Section 17.2.9) include:
- Human half-lives and other toxicokinetic data are not available for some PFAS found in drinking water and other environmental media.
- Currently available data indicate that reactive intermediates may form in the body from the metabolism of PFAA precursors to PFAAs. More studies are needed to understand the toxicologic significance of these intermediates.
- Data on absorption and toxicity of PFAS via dermal contact and inhalation are very limited, and more studies to characterize these exposure routes are needed.
- There are relatively few epidemiological studies of communities exposed to AFFF, PFOS, and/or other PFAS in drinking water. Although a number of studies of associations of PFAS with a variety of health effects have recently been reported for populations exposed to AFFF-contaminated drinking water in Sweden (Section 17.2.4), more such studies from other locations are needed.
- Additional toxicology data are needed for some PFAAs found in environmental media, including drinking water. In particular, very little toxicologic data are available for PFHpA, and no information was located for PFPeA.
- There is also a need for additional toxicological studies on the effects of PFAS mixtures in that although humans are exposed to multiple PFAS, information on toxicological interactions of PFAS is limited.
- Multigenerational studies of the reproductive and developmental effects of additional PFAS are needed.
- Chronic toxicity and carcinogenicity studies are currently available for only four PFAS (PFHxA, PFOA, PFOS, GenX), and are needed for PFHxS, PFNA, ADONA, and other PFAS to which humans may be exposed.
- The majority of the many thousands of PFAS, including those in commercial use, have very limited or no toxicity data. This is a critical data gap in health effects information for PFAS.
- Similarly, current NHANES biomonitoring in blood serum includes only 11 PFAS, primarily PFAAs, and breast milk biomonitoring data for these PFAS are limited. There is limited or no biomonitoring data in blood serum or breast milk for many other PFAS produced or used in the United States, some of which are known to be bioaccumulative in humans.
- Information on PFAS in powdered and prepared infant formula are extremely limited. Monitoring data for formula are needed to understand this potentially important source of exposure to infants.
- Little is known about how racial or socioeconomic differences may affect susceptibility to the adverse effects of PFAS exposure. Studies are needed to address this.
7.2 Ecological Toxicology
This section is organized around currently available toxicity information for invertebrates (aquatic/benthic/terrestrial), vertebrates (fish, birds, reptiles/amphibians, mammals), and plants. Toxicological data can be obtained from a general literature review as well as by querying of the USEPA Ecotox Database (USEPA 2023). As discussed below, this is an active area of research, and interested readers are encouraged to query the literature for updated research and reviews, such as the Environmental Toxicology and Chemistry Special Issue on Understanding Environmental Risk from Exposure to Per- and Polyfluoroalkyl Substances (PFAS; Vol 40, Issue 3, March 2021 https://setac.onlinelibrary.wiley.com/toc/15528618/2021/40/3); Argonne 2021; Zodrow et al. 2021; and Divine et al. 2020. There are also much data available for PFOS and PFOA toxicity that were used to derive the USEPA’s draft Aquatic Life Ambient Water Quality Criteria (US EPA 2022).
It is important to note that this section is not intended to represent an exhaustive review of PFAS ecological toxicology (referred to herein as “ecotoxicity”) studies. Ecotoxicity of PFAS is an area of active research, with new information emerging regularly. Toxicological effects of apical endpoints presented and discussed herein are generally those considered most relevant to ecological communities—mainly survival, growth, and reproduction. Both acute and chronic exposure studies are discussed in this section. Although data have been generated for other toxicological endpoints, these studies are not the focus of this section, but may occasionally be referenced. Application of these data in ecological risk assessment is discussed in Section 9.2
7.2.1 Introduction
Biomonitoring studies across a variety of organisms, habitats, and geographies show that certain PFAS can accumulate in wildlife and that exposures are occurring on a global scale (Reiner and Place 2015; Giesy and Kannan 2001[]). Therefore, it is important to understand how PFAS exposure and bioaccumulation may manifest in adverse effects, particularly as these effects relate to ecological communities. Information on bioaccumulation of PFAS is addressed in Section 6.5. This section provides an overview of resources available and some published toxicological data relating exposure of PFAS to toxic effects on aquatic, benthic, and terrestrial organisms, with the goal of broadening the reader’s understanding of known or potential effects in ecological systems, as well as highlighting areas where more data are needed. This information can also be applied for use in ecological risk assessments (ERAs), particularly because the ecological risk of PFAS is currently neither well understood nor uniformly assessed or regulated. However, the reader is encouraged to review the primary source literature from which cited ecotoxicity values have been derived to confirm and understand the basis and assumptions of the cited literature before using information obtained from this section in an ERA.
This review shows that ecotoxicity data are available for certain PFAS, particularly for PFOA and PFOS, with most studies focused to date on aquatic invertebrates. Although there are numerous studies on PFAS exposure in terrestrial vertebrates (for example, mammals, reptiles, birds), and ample toxicological studies in laboratory animals, there is, overall, relatively little to no ecologically relevant toxicity data for terrestrial vertebrates in the wild (though this is currently being investigated for avian receptors; see SERDP ER22-3202, https://serdp-estcp.org). Although some mechanistic studies have been conducted with aquatic organisms, little has been done with other organisms and even less has been done with different classes of PFAS in aquatic and terrestrial wildlife. However, see research funded under SERDP’s Statement of Need (SON): Improved Understanding of the Ecotoxicity of Mixtures of Per- and Polyfluoroalkyl Substances (ERSON-22-C1) for projects investigating mechanisms of PFAS toxicity and accumulation in aquatic and terrestrial receptors.
The focus of most ecotoxicity studies to date has been primarily on PFOS and PFOA. Therefore, most of the data discussed and summarized in this section are for those two compounds. However, data for other PFAS, including short-chain PFAS and precursors (Section 2.2), are also presented where available. Given the historical differences among older analytical methods and more recent advances in analyzing PFAS, the focus of the ecotoxicity studies covered in this review is generally on those published from approximately the year 2000 and later.
In general, single-chemical PFAS exposure studies indicate that the sensitivity of invertebrates to PFAS exposure can be chemical-specific and vary by organism and environmental factors (for example, see Lewis et al. 2022). Published studies of the toxicity of PFAS mixtures are available (Section 17.2.7.2), but the understanding of PFAS mixtures toxicity remains uncertain at this time. Risk assessment implications of exposure to mixtures are discussed further in Section 9.2.1.3. There is a paucity of field studies for avian and mammalian wildlife species, and confounding factors such as the co-occurrence of other stressors (other pollutants, physical stressors, etc.) makes it difficult to definitively associate PFAS exposure with adverse outcomes (Custer 2021). There are a handful of avian studies on multiple species that investigate egg hatching outcomes and potential correlations to PFAS exposures (Custer et al. 2014; Groffen et al. 2019; Tartu et al. 2014), and field-based effects studies on mammals are difficult to find (ECCC 2018). However, laboratory animal studies (see Section 7.1) suggest potential relationships between PFAS tissue concentrations and immunological, hematological, liver, kidney, and reproductive effects (DeWitt 2015; ECCC 2018).
Data from biomonitoring studies (see Section 5.5) indicate that PFAS exposure is occurring in wildlife; however, the lack of toxicity data for this group of organisms represents a significant data gap. This highlights the need for additional study of this class of compounds in general, as well as the need for expansion of toxicity studies to a larger group of PFAS and to a greater variety of taxa, and for field studies that may assess population-level effects.
Relative aquatic toxicity for PFAS is discussed in the following sections using descriptive criteria developed by the USEPA within their Design for the Environment Program for the Alternatives Assessments and the Safer Choice Program (USEPA 2011[]; USEPA 2015). These criteria are expressed as relative toxicity based on effects concentrations ranging from less than 0.1 mg/L (very high toxicity) to greater than 100 mg/L (low toxicity); criteria are provided in Table 7-2.
Table 7-2. Aquatic toxicity classification criteria (USEPA 2011) (in mg constituent/L water)
| Toxicity | Very High | High | Moderate | Low | Very Low |
|---|---|---|---|---|---|
| USEPA: Aquatic Toxicity (Acute, LC50) | <1.0 | 1–10 | >10–100 | >100 | NA |
| USEPA: Aquatic Toxicity (Chronic, LOEC) | <0.1 | 0.1–1 | >1–10 | >10 | NA |
7.2.2 Invertebrates
7.2.2.1 Aquatic
Aquatic invertebrates may be exposed to PFAS by direct contact with PFAS in the water column, as well as via the diet, including trophic transfer and particle ingestion. There are more toxicity data available for PFOS than for other PFAS. PFAS have a very wide range of toxicities to aquatic organisms under acute exposure scenarios following the USEPA Hazard Criteria (Table 7-2), but overall, they would be classified as having moderate to low toxicity to invertebrates. One exception with this generalization is that of mussel exposures to PFOS and PFOA in the marine environment, where no effect was seen at 0.00001 mg/L but a LOEC was reported at 0.0001 mg/L (Fabbri et al. 2014); this would result in classification as a very high hazard using the USEPA Hazard Criteria. With the current body of literature, the sensitivity of marine invertebrates to PFOS and PFOA appears equivocal.
Given that PFAS are persistent pollutants, chronic exposure scenarios are most relevant for aquatic receptors. Importantly, compared to acute studies, there are relatively few chronic studies in aquatic invertebrates. Most chronic effects data are for PFOS and PFOA. Life cycle tests with multiple taxa have been conducted to evaluate the chronic toxicity of PFOS to freshwater aquatic invertebrates. The chironomid (Chironomus tentans) is currently reported as having the greatest sensitivity to chronic exposure, with reduced total emergence reported at 0.0023 mg PFOS/L (MacDonald et al. 2004). More recent studies, including McCarthy et al. (2021), show impairment on survival in the chironomid (C. dilutus ) at even lower concentrations, with EC10 and EC20 values in 20-day tests for PFOS at 1.4 and 1.7 ug/L, respectively. Studies by Bots et al. (2010) and Van Gossum et al. (2009) indicated that damselflies (Enallagma cyathigerum) may also be similarly sensitive to PFOS, with NOEC and LOEC values of 0.01 and 0.1 mg/L, respectively. In the marine environment, a life cycle toxicity test with the saltwater mysid (Mysidopsis bahia) yielded a NOEC of 0.24 mg PFOS/L based on growth and number of young produced (Drottar and Krueger 2000).
Some PFAS may potentially cause adverse effects in aquatic invertebrates that span across multiple generations. Marziali et al. (2019) evaluated generational effects in chironomids (C. riparius); each generation was exposed to a nominal concentration of 0.01 mg/L of PFOA, PFOS, and PFBS; all treatments showed reduced growth in at least several generations, with no observed induced tolerance to the studied PFAS. However, potential effects at the population level were not demonstrated in this study based on similar population growth rates between treatments and controls, suggesting that toxicity risk to an ecosystem is unlikely (Marziali et al. 2019).
Benthic Organisms and Sediment Toxicity
Toxicity to benthic (sediment-dwelling) organisms is generally the result of exposure to the chemical in overlying water, sediment, and porewater, including trophic transfer and sediment particle ingestion (Zareitalabad et al. 2013). There are relatively few published sediment toxicity studies on PFAS exposure to benthic invertebrates compared to those for aquatic invertebrates. The UK Environment Agency (2004)[] provided a freshwater sediment screening value of 0.0067 mg/kg (wet weight), based upon a predicted no-effect concentration (PNEC) of 2.5 µg/L and a river sediment Kd of 8.7 L/kg. Note, however, that sediment screening values based on Kd may not be applicable across all sites. Section 9.2 (Ecological Risk Assessment) discusses application of Kd in deriving sediment screening values.
Additionally, Bakke et al. (2010) provided PFOS concentration ranges for marine sediment quality classified as background, good, moderate, bad, and very bad. The PFOS threshold for “good” sediment, for which no toxic effects are expected, was reported as 0.22 mg/kg. This value, however, is based on an aquatic PNEC of 72 µg/L derived from a limited data set and an unspecified Kd value, and thus is not a reliable concentration with which to predict toxic effects. More recently, Simpson et al. (2021) conducted a multimedia acute and chronic study on a variety of marine/estuarine invertebrates (including an amphipod, a copepod, a crab, and two species of bivalve) that included PFOS-spiked sediment toxicity tests. Simpson et al. (2021) found that PFOS significantly decreased survival and/or reproduction for the amphipod (Melita plumulosa) at sediment concentrations of 29 mg/kg or greater. Although they did not identify a relationship between toxicity and PFOS concentrations in sediment, they observed a strong relationship among toxicity, organic carbon content of sediments, and dissolved PFOS concentrations in the overlying water of the test vessel. These results suggested that the dissolved fraction of PFOS in water is likely a key contributor to sediment toxicity. Based on sediment concentrations normalized to 1% organic carbon (1%OC), the authors derived an LC10 of 132 mg/kg (1%OC) and LC50 of 150 mg/kg (1%OC) for PFOS, and EC10, EC20, and EC50 of 21, 35, and 89 mg/kg (1%OC), respectively, for reproductive effects (Simpson et al. 2021). The focus of Simpson et al. (2021) was on the amphipod, but this publication also provided data for additional marine species. The authors also developed sediment thresholds using Kd and species sensitivity distributions from water exposures.
Laboratory-controlled freshwater and marine sediment toxicity tests for PFAS are sparse. With so few studies available and with variability in test organisms and testing methods, it is difficult to define PFAS toxicity thresholds for benthic organisms or to determine if benthic organisms are similarly sensitive to PFAS compared to other aquatic invertebrates. However, benthic organism toxicity thresholds do not need to be limited to just invertebrates, as shown by Simpson et al. (2021), who developed PFOS thresholds from a species sensitivity distribution (SSD) generated from published data on other types of species using a Kd approach.
7.2.2.2 Terrestrial Invertebrates
Compared to aquatic invertebrates, there are fewer studies on the effects of PFAS on terrestrial invertebrates (i.e., invertebrates living in terrestrial habitats). Brignole et al. (2003), whose study was summarized in Beach et al. (2006), summarized results of acute oral and dermal studies of PFOS conducted on the honeybee (Apis melifera), although the dose was reported in terms of mass of PFOS per bee, which may not be relevant for evaluating ecological risks. However, these studies, when converted to a dose per kilogram of food (2 mg PFOS per kg sugar solution), suggested that PFOS was “highly toxic” to honeybees, as defined by the International Commission for Bee Botany. Mommaerts et al. (2011) identified in a chronic oral dosing study on the bumblebee (Bombus terrestris) an LC50 of 1.01 mg PFOS/L sugar water and noted that PFOS exposure caused detrimental reproductive effects (decreased ovarian size).
Effects on fecundity from exposure to various PFAS have been shown to carry down through multiple generations in the roundworm (Caenorhabditis elegans). Tominaga et al. (2004) conducted a multigenerational study in C. elegans exposed to PFOA, PFOS, and PFNA, finding that concentrations orders of magnitude lower than those causing lethality decreased worm abundance, and that effects were observed even in the fourth generation. Other studies have evaluated the mechanisms of PFAS toxicity. Xu et al. (2013) indicated that exposure to PFOS induced oxidative stress and DNA damage in the earthworm, Eisenia fetida. Stylianou et al. (2019) evaluated food chain transfer of PFOS-treated Escherichia coli to C. elegans and noted distinct gene expression profiles associated with development, innate immunity, and stress response.
With regard to soil invertebrate toxicity testing, studies (while few in number) suggest a low to moderate toxicity of PFOS and PFOA, with toxicity generally occurring on a parts per million scale. These studies have mainly focused on the earthworm Eisenia fetida. Sindermann et al. (2002) conducted a 14-day chronic soil study on E. fetida with PFOS and identified a NOEC of 77 mg PFOS/kg soil, a LOEC of 141 mg/kg, and an LD50 of 373 mg/kg. Other chronic earthworm studies indicated toxic concentrations of a similar magnitude, with LC50s ranging from 84 mg/kg–447 mg/kg (Mayilswami et al. 2014; Zareitalabad et al. 2013). The Norwegian Pollution Control Authority NPCA (2006), as reported in Danish Ministry of the Environment (2015), conducted acute soil toxicity tests in E. fetida, looking at reproductive endpoints for PFOA, PFOS, and the short-chain 6:2 fluorotelomer sulfonate (6:2 FTS). Results of this study indicated that, overall, the evaluated PFAS exhibited a moderate-high toxicity. Reproductive effects (decreased number of cocoons, decreased hatchability, and decreased number and weight of juveniles) for PFOS and PFOA were noted. 6:2 FTS toxicity was found to be less than that for either PFOS or PFOA in the same study. Karnjanapiboonwong et al. (2018) conducted a 21-day soil study with E. fetida on bioaccumulation, mortality, and weight loss with PFBS, PFHxS, PFNA, and PFHpA and generally observed no effects at soil concentrations below 100 mg/kg in comparison with the controls. Importantly, the authors report tissue concentrations following exposures to PFBS, PFHxS, PFNA and PFHpA thus indicating potential for trophic transfer from soil to higher level organisms (Karnjanapiboonwong et al. 2018).
The limited amount of terrestrial invertebrate data presents a data gap; additional toxicity studies are needed to better characterize ecotoxicological effects in this group of organisms. Additionally, it will be important to understand how field/soil conditions (for example, organic carbon content, pH, etc.) may influence toxicity. For example, Princz et al. (2018) found that PFOS toxicity for two different species of soil invertebrates was approximately two to four times greater when organisms were tested on sandy loam versus clay loam soils.
7.2.3 Vertebrates
The following sections describe available toxicity data for vertebrate species, including fish, amphibians/reptiles, birds, and mammalian wildlife.
7.2.3.1 Fish
Acute freshwater LC50 values based on survival for PFOS range from 7.8 to 22 mg/L for Rainbow trout (Oncorhynchus mykiss), to 9.1 mg/L for fathead minnow (Pimephales promelas) (Robertson 1986; Palmer, Van Hoven and Krueger 2002).
There are relatively few chronic PFOS studies in fish, but (Drottar and Krueger (2000)) calculated a chronic NOAEL based on early life stage mortality in Pimephales promelas to be 0.29 mg/L. Palmer, Van Hoven and Krueger (2002) also calculated an acute NOAEL of 6.3 mg/L for Oncorhynchus mykiss. Saltwater acute values based on survival for Oncorhynchus mykiss were calculated to be 13.7 mg/L.
Other than PFOS, there are limited aquatic ecotoxicity data for other PFAS. Within the summary data presented here, acute exposure durations were for 6 days. One study was noted that investigated the chronic toxicity of PFNA following a 180-day exposure; the LOEC ranged from 0.01-1 mg/L depending on the endpoint (Zheng et al. 2011).
7.2.3.2 Amphibians/Reptiles
There are relatively limited toxicity data available for PFAS effects on amphibians, including several studies on various species of frogs; no studies on reptiles were found in the literature search. The data available for PFOS and PFOA show a wide range of effects-based concentrations.
More amphibian data are available for PFOS in comparison to other PFAS, and indicate mortality generally tends to occur at levels of 10 mg/L or higher, whereas nonlethal effects (for example, growth; EC10) may occur at approximately 1–2 mg/L (that is, moderate to high toxicity) or lower (Ankley et al. 2004; Yang et al. 2014; Fort et al. 2019). However, more recent studies suggest chronic toxicity may occur at levels lower than 1 mg/L. Flynn et al. (2019), for example, found a 72-day LOEC in the American bullfrog (Lithobates catesbeiana) of 0.29 mg/L based on developmental effects (reduction in snout vent length).
Ankley et al. (2004) conducted a 5-week study on PFOS toxicity in the northern leopard frog (Rana pipiens) and observed that LC50s decreased with increasing test duration time; LC50s ranged from 12.5 mg/L at 1 week to 6.2 mg/L at 5 weeks. This study also anecdotally noted the presence of kinked tails, as well as a delayed time to initial metamorphosis and differences in limb bud and foot paddle emergence in the 1, 3, and 10 mg/L groups. A PFOS study, based on a 3M study reported in OECD (2002) on another frog species, African clawed frog (Xenopus laevis), suggested toxicity at concentrations of similar magnitude to those observed in the Ankley study, and identified inhibition of growth and malformation during development.
PFAS exposure has also been shown to affect the thyroid, and hence development, in amphibians (for example, Cheng et al. 2011; Flynn et al. 2022). A study by Cheng et al. (2011) that exposed African clawed frog (X. laevis) tadpoles to PFOS at concentrations ranging from 0.1 to 100 ug/L in water found up-regulation of several thyroid hormone–regulated genes, suggesting possible thyroid disruption. Flynn et al. (2022) conducted a chronic study that evaluated sublethal developmental effects on R. pipiens, the American toad (Anaxyrus americanus), and the eastern tiger salamander (Ambystoma tigrinum), following exposure up to 30 days to 10, 100, and 1,000 ug/L of PFOS, PFOA, PFHxS, and 6:2 FTS). They found a LOEC of 10 ug/L based on reduced body mass for all four PFAS in anurans, and PFOA, PFHxS, and 6:2 FTS in the salamander.
Amphibian studies suggest that PFOS may be less toxic than certain other PFAS ( Yang et al. 2014 and Abercrombie et al. 2021) Brown, Flynn, and Hoverman (2021) found increased trematode infection in the northern leopard frog resulting from exposure to 0.01 mg/L PFHxS, whereas no effect was observed for PFOS (however, no such effect was noted in the higher PFHxS treatment dose of 0.1 mg/L; the authors postulated that this could potentially be due to adverse effects of PFHxS to the trematode itself).
Many of the older amphibian studies focused on early life aquatic exposures. More recent studies suggest that amphibians may be even more sensitive to PFAS if one looks at later life stages; nonlethal effects other than survival; growth and reproduction; terrestrial exposure routes; or multiple exposure media. Abercrombie et al. (2021) indicated LOECs ranging from 0.05-0.12 mg/L for a toad, frog, and salamander exposed to PFOS, PFOA, or PFHxS in a soil substrate. Flynn et al. (2021) found enhanced PFOS and PFOA toxicity in a spiked sediment outdoor mesocosm study, suggesting that evaluating only aquatic exposures may underestimate toxicity from exposure to PFAS in multiple media, which is more representative of actual field conditions.
7.2.3.3 Birds
There are currently a limited number of published laboratory studies available that address PFAS toxicity in avian wildlife species (Newsted et al. 2006; Newsted et al. 2005; Newsted et al. 2007; Newsted et al. 2008; Dennis et al. 2020; Bursian et al. 2021). The northern bobwhite quail (Colinus virginianus) and the mallard duck (Anas platyrhynchos) were exposed to PFOS (Newsted et al. 2005; Newsted et al. 2006; Newsted et al. 2007) or PFBS (Newsted et al. 2008) via the diet. The LD50s reported following acute exposure to PFOS are 61 mg/kg-bw/d and 150 mg/kg-bw/d for the northern bobwhite quail and the mallard, respectively (Newsted et al. 2006). In a separate chronic dietary study, Newsted et al. (2007) found that a feed concentration of 10 mg PFOS/kg resulted in an average daily intake (ADI) LOAEL of 0.77 mg/kg-bw/d based on increased liver weight in female quail. A feed concentration rate of 50 mg/kg resulted in an ADI LOAEL of 6.4 mg/kg-bw/d based on lethality in mallards (Newsted et al. 2007).
More recent avian studies on the northern bobwhite (Dennis et al. 2020) and Japanese quail (Coturnix japonica) (Bursian et al. 2021) evaluated dietary effects of PFOS, PFOA, PFHxS, and the AFFF formulations from 3M and Ansul. Bursian et al. (2021) estimated average daily doses causing 50% mortality (ADD50) at 5 days’ exposure ranging from 38 mg/kg-d for PFOS (≥98% chemical grade) to 130 mg/kg-d for PFOS in feed spiked with 3M AFFF, which is a product formulation that is a mixture of fluorinated and other compounds (LC50 of 351–467 mg/kg of feed, respectively). More information about AFFF is included in Section 3. This subacute study also evaluated effects resulting from a mixture of PFOS and PFOA; the authors suggested that these results showed potential additive effects, and a relative potency of PFOA approximately half that of PFOS (Bursian et al. 2021). Dennis et al. (2020) evaluated chronic toxicity associated with PFOS and a mixture of PFOS and PFHxS in drinking water on the northern bobwhite quail. Reduced body weight in females and impaired hatching success were observed at doses lower than those used in the Newsted study (Newsted et al. 2007), deriving a chronic toxic dose of 0.0031 mg PFOS+ PFHxS/kg-bw/d and 0.00245 mg PFOS/kg-bw/d, respective to the previously mentioned endpoints.
Some egg injection studies suggest exposure to PFOS may adversely affect chick development during incubation. For example, Molina et al. (2006) found that eggs injected with varying doses of PFOS had a lower rate of hatching success and had noted pathological changes in the liver in the leghorn chicken. However, there is some concern regarding the use of these data in risk assessments due to issues related to the method of exposure and other methodological issues that can influence the outcomes of the studies. Although these studies are useful for evaluating mechanisms and creating structure-activity relationships, they may not be appropriate for direct application in risk assessments.
Quail and mallard appear less sensitive to PFBS relative to PFOS. Acute dietary exposure to PFBS resulted in NOAELs of 3,160 and 5,620 mg PFBS/kg-feed for the northern bobwhite quail and mallard, respectively, for the lethal endpoint; these feed concentrations are equivalent to an ADI of 774 mg/kg-bw/d in quail and 2,190 mg/kg-bw/d in mallards (Newsted et al. 2008). A NOAEC for northern bobwhite quail reproduction following chronic dietary exposure to PFBS was reported at 900 mg/kg-feed, equivalent to an ADI of 87.8 mg/kg-bw/d (Newsted et al. 2008).
Although there are few PFAS laboratory toxicity studies for birds, there are even fewer effect-based field studies. Custer et al. (2012) and Custer et al. (2014) evaluated PFOS exposure in tree swallows (Tachycineta bicolor), identifying a negative association between PFOS concentration in eggs (~150 ng/g-ww) and hatching success. One issue with the findings as suggested by the authors is that the greatest observed effects on hatching were typically found in areas that likely have co-contamination issues (PCBs, PAHs, mercury); however, the influence of these other contaminants is still not clear. This issue of effects from co-contaminants came to light when Custer et al. (2019) conducted a field study evaluating the effects of PFAS on tree swallows in a more isolated area with known PFAS impacts (Clark’s Marsh near the former Wurtsmith Air Force Base, MI); other potential contaminants were at or below regional anthropogenic background levels. This study resulted in mean PFOS egg concentrations of 662 ng/g-ww, and overall hatching success was not impacted. Groffen et al. (2019) conducted a field study in great tits (Parus major) near a former fluorochemical plant. They reported median egg concentrations of 48,056 ng/g-ww for PFOS, 18 ng/g-ww for PFOA, and 315 ng/g-ww for PFDS. The authors concluded that reduced hatching success in the birds was associated with a mixture of PFAS (PFOS, PFDS, PFDoDA, PFTrDA, and PFTeDA) (Groffen et al. 2019). Taken together, a linear relationship between PFAS exposure and potential effects in avian species remains uncertain; this highlights the importance of considering co-exposure of common environmental contaminants and PFAS mixtures when reviewing field studies.
Lastly, indirect effects, such as reduction in a local food source resulting from a PFAS release, may potentially affect bird populations. de Vries et al. (2017), for example, found a decline in abundance of flamingos following a release of fire-fighting foams containing PFOS and other PFAS to a salt lake; the authors postulated that this decline was potentially related to a reduction in prey item abundance due to PFOS toxicity.
7.2.3.4 Mammalian Wildlife
PFAS exposure to wildlife is occurring on a global scale and across a variety of habitats (Reiner and Place 2015; Giesy and Kannan, 2001). Wildlife may accumulate PFAS from direct exposure to air, dust, water, soil, and sediments, as well as through diet. Maternal transfer of PFAS is also a relevant exposure route, as these compounds have been shown to cross the placenta (Gronnestad et al. 2017; Houde et al. 2006). PFAS have also been shown to biomagnify, so higher trophic level predators have higher PFAS levels in tissues compared with prey items (Reiner and Place 2015). Of the PFAS analyzed in wildlife exposure studies, PFOS is the one most frequently detected, and at the highest concentrations, in tissue samples (Reiner and Place 2015). Concentrations in tissue have also been observed to vary with age, sex, and species (DeSilva et al. 2021).
Given the widespread occurrence of PFAS in wildlife, it is important to understand if such exposure manifests in adverse effects and ultimately how exposure may impact wildlife populations. Laboratory animal models show that, in general, PFAS are readily absorbed and distributed among protein-rich tissues (liver, serum, kidney) in mammals, and that certain PFAS (particularly long-chain compounds) have a relatively long half-life in the body. Toxicity tests on laboratory mammals (mice, etc.) have shown that exposure to PFAS may result in adverse effects on the hepatic, endocrine, and immune systems; development; and certain types of cancers, as discussed in Section 7.1.4.
Based on the findings from mammalian toxicity studies in laboratory animals, one might expect to find similar effects in mammalian wildlife (at similar exposure levels). Laboratory studies focusing on growth, reproduction, and survival effects on laboratory mammals provide data to support the development of toxicity reference values for use in ERA of wildlife species. NOAELs and LOAELs can be derived from these studies for use in ERA (as further discussed in Section 9.2, Ecological Risk Assessment), but these values should be used with caution and understanding of their associated uncertainty. Many of these studies may have also included other endpoints, such as systemic or metabolic endpoints, that are not typically used for ERA and may demonstrate effects at lower doses than the growth, reproduction, and survival effects.
Although there are numerous studies evaluating toxicity of PFAS in laboratory animals (as discussed in Section 7.1.4), and there are numerous exposure studies in mammalian wildlife, very few studies have evaluated PFAS toxicity with respect to wildlife exposures. The studies that have been conducted typically evaluated relationships between the concentrations of a small number of PFAS in various protein-rich biological media (for example, blood serum, liver) and expression of select biomarkers. One study on sea otters related concentrations of PFOA and PFOS in liver tissue to health condition and possible immune effects (Kannan, Perotta and Thomas 2006). Table 7-3 summarizes these studies.
Table 7-3. Summary of PFAS toxicity studies in mammalian wildlife
| Species | Summary of Findings | Reference |
|---|---|---|
| Sea otter
Enhydra lutris |
Higher PFOS/PFOA concentrations in liver samples found in diseased otters versus nondiseased group | Kannan, Perrotta and Thomas 2006 |
| Bottlenose dolphin
Tursiops truncatus |
Significant positive associations between serum total PFAS concentrations and multiple immunological, hematopoietic, renal, and hepatic function endpoints | Fair et al. 2013 |
| Wood mouse
Apodemus sylvaticus |
Significant positive relationship between liver PFOS concentration and hepatic endpoints (relative liver weight, microsomal lipid peroxidation level); significant negative association with serum alanine aminotransferase (ALT) activity | Hoff et al. 2004 |
| Wild pig
Sus scrofa |
No significant correlation between PFAS liver concentrations and multiple blood, hepatic, and immunological endpoints, whereas significant correlations were observed for other pollutants (for example, dioxin-like compounds, PCBs, organohaline pesticides) | Watanabe et al. 2010 |
It is important to note that while certain associations have been observed between PFAS concentrations and various immunological, hematopoietic, renal, and hepatic function biomarkers, these associations are not necessarily indicative of actual impairment to an individual organism or within a larger population.
Perhaps one of the biggest challenges with wildlife toxicity studies is that wildlife are exposed to multiple chemical, biological, and physical stressors, making it difficult to determine whether noted effects are directly related to PFAS, to other stressors, or to a combination of stressors. The accumulation of other types of POPs, such as PCBs, dioxins, and pesticides, and metals such as mercury, in wildlife has been well established and, in some studies, correlated body burden to adverse effects. Arctic mammal studies have reported relationships between organohalogen exposure and endocrine disruption, reduced immune function, and adverse effects on the liver and other organs (Letcher et al. 2010). Numerous nonchemical environmental factors such as climate change, habitat loss, and seasonal availability of food may also confound toxicity studies, making it difficult for field studies to discriminate those effects related solely to PFAS. As an example, Watanabe et al. (2010) found no association between PFAS levels and a variety of biomarkers in wild pigs, whereas the study found significant positive associations between these parameters and other types of contaminants (for example, PCBs) that were also detected in liver tissue samples.
Currently, there are few data points available for mammalian wildlife, and the current literature focuses on bioaccumulation and specific endpoints that may not be ecologically relevant, as discussed above. Additionally, bioaccumulation studies have traditionally focused on protein-rich tissues such as liver or blood serum because PFAS preferentially bind to proteins; because PFAS could also be present in other tissues, focus on only a subset of tissues can potentially underestimate the total body burden of PFAS. Thus, exposure cannot be fully characterized from these studies, and pinpointing correlations between target organ or whole-body effects and PFAS exposure is not possible at this point in time. A better understanding of mammalian exposures to the broad spectrum of PFAS, precursor compounds, and mixtures of PFAS, as well as other environmental contaminants, is critical in advancing this field of study. Given the challenges with conducting field studies, this information could be obtained in part through more robust dosing studies in mammals that are representative of various wildlife taxa, and on toxicological endpoints that are directly relevant to population-based effects; however, more field studies are also needed to confirm laboratory models. Groups such as the U.S. Department of Defense’s Strategic Environmental Research and Development Program (SERDP) and the Environmental Security Technology Certification Program (ESTCP) have recently identified such critical data needs (SERDP-ESTCP 2017).
7.2.4 Plants
The following sections describe available toxicity data for aquatic and terrestrial plants.
.
7.2.4.1 Aquatic Plants
Data on the toxic effects of PFAS on aquatic plants are limited, with available studies focusing on PFOS included in USEPA Ecotox Database (USEPA 2023). The acute toxicity (EC50s) of PFOS to aquatic plants (such as Selanastrum and Lemna species) generally ranges from roughly 31 to 108 parts per million (mg/L), with NOEC values from the same studies being approximately 7–30 mg/L; (Boudreau et al. 2003; Sutherland and Krueger 2001; Drottar and Krueger 2000). Chronic effects (EC50s) were found to be similar to acute values, but varied over a wide range, depending on species and endpoint (2–305 mg/L), with NOECs from the same studies ranging from 0.3 to 11.4 mg/L (Hanson et al. 2005; Boudreau et al. 2003; Desjardins et al. 2001, Desjardins et al. 2001; Desjardins et al. 2001).
7.2.4.2 Terrestrial Plants
There are limited PFAS toxicity data for terrestrial plants; a review of the literature yielded only a few soil phytotoxicity studies. Brignole et al. (2003) evaluated PFOS exposure (21 days) on a variety of crop plants (alfalfa, onion, ryegrass, soybean, tomato, flax, and lettuce) using emergence, survival, and shoot height and weight as endpoints, and demonstrated effects occurring at concentrations ranging from 57 mg/kg to over 1,000 mg/kg. Other studies (Li 2009; Zhao et al. (2011)) conducted on both PFOS and PFOA on multiple crop plants found a wide range of toxicity among species and also observed a range of toxicity within species (specifically, Brassica rapa chinensis). The most sensitive species may be Triticum aestivum where the 30-day NOEC reported was 1 mg/kg (Zhao et al. 2014). Toxicity may also be moderated by soil characteristics; for example, Zhao et al. (2011) showed that the amount of organic matter in soil significantly influenced toxicity, where higher organic carbon content decreased both accumulation of PFOA and PFOS and phytotoxicity. Additionally, PFAS chain length may affect uptake of PFAS into plant tissue; see Section 5.6 for further discussion.
7.2.5 Uncertainties and Conclusions
This section presented ecotoxicological information with the intent of providing the reader with an overview of the types of organisms and ecotoxicity studies available for PFAS in the current literature (as of 2023). This section also presented available information about the ranges of concentrations of PFAS (notably, PFOS) in soil, sediment, and water that have been associated with adverse effects. In summary, ecotoxicity studies demonstrate a wide range of effects concentrations across the various terrestrial and aquatic biota. In general, aquatic invertebrates appear to be more sensitive to PFOS and other PFAS than their terrestrial counterparts. Differences in species sensitivities, analytical methods, environmental substrate, test conditions, and reproducibility of results make it difficult to generalize overall effects, and some species may be more or less sensitive than others.
Although there are numerous studies on the toxicity of select PFAS to aquatic invertebrates, these studies are generally limited to a very small number of PFAS (typically PFOS, and to a lesser extent, PFOA). Because PFAS represent a broad spectrum of compounds, it is important to expand ecotoxicity studies to evaluate additional PFAS, including short-chain and precursor compounds, as well as “next generation” replacement compounds. Recently, Jones et al. (2022) published a compilation of acute aquatic toxicity following exposures to “next-generation” foams and one reference foam (short-chain PFAS AFFF). Of the 14 species tested, the short-chain PFAS AFFF was least toxic in comparison to the next-generation foams in 11 taxa following acute exposure. In cross-species comparison for the short-chain PFAS AFFF lethality data, the mud snail (T. obsoleta) and the freshwater amphipod (H. azteca) were the most sensitive, and a variety of amphibian species were the least sensitive (Jones et al. 2022). More data are anticipated from these authors on chronic exposures to a subset of these species with projects funded under the SERDP Statement of Need ERSON-20-A1, which is aimed at quantifying the potential ecotoxicity of fluorine-free surfactant foam formulations (www.serdp-estcp.org). Other studies show that some of the short-chain replacements, such as GenX, are more toxic than PFOS-based foam formulations (Conley et al. 2022).
Importantly, the available studies on foam formulations and individual PFAS exposures indicate a wide range of effects levels for PFAS in aquatic invertebrates, suggesting a level of complexity that has not yet been adequately assessed.
Significantly fewer toxicity studies are available for other groups of aquatic or benthic organisms, and few to no studies are available for avian or mammalian wildlife or plants, presenting a significant gap in our understanding of how the widespread presence of PFAS in the environment may be affecting ecological communities. Additional (or any) data on toxicological endpoints most relevant to community-level effects, such as survival, growth, and reproduction, will be extremely beneficial in understanding potential ecological impacts.
Updated September 2023.