16 Surface Water Quality
The purpose of this section is to support the PFAS Technical and Regulatory Guidance Document (PFAS Guidance Document) users (state and federal environmental staff, stakeholders, project managers, and decision makers) to gain a working knowledge of the current state of PFAS science and practice for surface water quality. The section does not include calculated criteria, rather it provides available information and references that can then be used to support development or review of criteria or guidance values to protect surface water quality.
| Section Number | Topic |
|---|---|
| 16.1 | Introduction |
| 16.2 | Protection of Human Health |
| 16.3 | Protection of Biota |
| 16.4 | Sampling and Analysis |
| 16.5 | Surface Water Foam |
| 16.6 | Effluent Limits for PFAS |
| 16.7 | Surface Water/Groundwater Interaction |
16.1 Introduction
This section provides information to help fill in the gaps related specifically to concerns with PFAS-impacted surface water and associated receptors. Several states have expressed a need for technical information to support the development of surface water quality criteria (WQC) or guidance values for uses other than drinking water, including but not limited to habitat for fish and other aquatic life. USEPA (2020) developed recommendations and provided derivation methods for surface water criteria “for determining when water has become unsafe for people and wildlife using the latest scientific knowledge.” States and tribal governments may, and sometimes do, develop their own numerical criteria. To protect human health, the states and tribal governments may also establish water body–specific fish consumption advisories for certain aquatic species. These advisories are recommendations and not enforceable. This section provides technical information regarding PFAS in surface water that individual states and tribal governments can consider when developing criteria according to their own processes and policies. This information focuses on two main issues. First is the protection of human health from a variety of potential exposures to PFAS in surface water, such as drinking water, consumption of fish and other aquatic species, and from recreational activities. Second is the protection of biota, based on available ecotoxicity data, bioaccumulation and concentration factors, and aquatic-dependent wildlife considerations, among others.
USEPA has published draft national recommended aquatic life criteria for PFOA and PFOS in freshwater for public comment (USEPA 2022, 2022) and a fact sheet for the criteria (USEPA 2022). In addition, USEPA had published their responses to external peer reviews of the draft criteria (USEPA 2022, 2022). USEPA has also issued two memoranda for National Pollution Discharge Elimination System (NPDES) permits and PFAS. One addresses PFAS under USEPA-issued NPDES permits for wastewater and stormwater discharges (USEPA 2022) and the other discusses PFAS in state-issued NPDES permits and pretreatment programs (USEPA 2022). As of the writing of this document, there are few formally established surface water criteria for any PFAS that are protective of uses of surface water other than as drinking water. Florida, Minnesota, and Michigan are examples of states that have aquatic life protection values (see the Water and Soil Regulatory and Guidance Values Table Excel file).
In addition to the well-established issues associated with PFAS in surface water, such as use of the surface water as a source of drinking water and accumulation of PFAS in biota, this section also includes a discussion of a relatively new issue related to surface water: PFAS-containing foam. Investigations in at least three states—Minnesota, Michigan, and Wisconsin—have found that concentrations of PFAS in surface water, or discharging to surface water, at sufficient levels can generate foam on surface water. That foam acts to remove PFAS from the water column, but also acts as a secondary source of PFAS as the foam leaves the surface water and is deposited in terrestrial or other aquatic locations.
16.1.1 Beneficial Uses
One of the first steps in developing the criteria is to determine the beneficial uses that are to be protected for the water body of concern. These have usually been developed by the state organization responsible for water quality and/or water resources for the state. That regulatory agency assesses potential beneficial uses and assigns appropriate designated uses for a water body. As examples, in Massachusetts this task is the responsibility of the Massachusetts Department of Environmental Protection, while in California it is under the purview of the nine Regional Water Quality Control Boards that establish potential beneficial and designated uses for the water bodies for each region. The process for establishing these beneficial uses in most instances follows protocol developed by USEPA (2020).
With the potential adverse health effects associated with the ingestion of certain levels of various PFAS, negative impacts on beneficial uses of surface water may occur. Table 16.1 provides a fairly comprehensive list of potential beneficial uses for surface water. This table is a compendium taken from the Water Quality Control Plan (Basin Plan) for the California Regional Water Quality Control Board, Central Valley Region, Fourth Edition, Sacramento and San Joaquin River Basins (RWQCB-CVR 2016). Different jurisdictions use different lists of beneficial uses. The list in Table 16-1 is used for illustration. For an evaluation of PFAS, the list may be substantially reduced in order to focus on those that are appropriate, as the presence of PFAS may not have an impact on a particular use (for example, navigation). In the table the list has been reduced by separating the beneficial uses that may be impacted by PFAS (light blue) and those that are not likely to be (light green). The list can be further reduced by combining several of the uses that evaluate similar issues, such as aquatic toxicity. As an example, the beneficial uses listed as WARM, COLD, EST, WILD, BIOL, and RARE (see Table 16.1) are each designed to protect aquatic species that have a range of attributes. The beneficial use for shellfish could be added to that grouping by expanding the evaluation under it to include benthic organisms and sediment quality.
Table 16-1. Beneficial Uses
Source: Adapted from RWQCB-CVR (2016)
| Beneficial Use | Description | Does PFAS Have Impact on Use? Covered in this Section | |
|---|---|---|---|
| 1. | Municipal and Domestic Supply, Use as Drinking Water (MUN) | Uses of water for community, military, or individual water supply systems, including, but not limited to, drinking water supply. | Yes |
| 2. | Agricultural Supply (AGR) | Uses of water for farming, horticulture, dairy operations, or ranching. | Yes |
| 3. | Primary Water Contact Recreation (REC-1) | Uses of water for recreational activities involving body contact with water, where ingestion of water is reasonably possible. | Yes |
| 4. | Groundwater Recharge (GWR) | Uses of water for natural or artificial recharge of groundwater. | Yes |
| 5. | Commercial and Sport Fishing (COMM) | Uses of water for commercial or recreational collection of fish, shellfish, or other organisms. | Yes |
| 6. | Aquaculture (AQUA) | Uses of water for aquaculture or mariculture operations. | Yes—not all components covered under other beneficial uses—harvesting of aquatic plants for human consumption |
| 7. | Warm Freshwater Habitat (WARM) | Uses of water that support warm water ecosystems. | Yes—combine with COLD |
| 8. | Cold Freshwater Habitat (COLD) | Uses of water that support cold water ecosystems. | Yes—combine with WARM |
| 9. | Estuarine and Marine Habitats (EST) | Uses of water that support estuarine and marine ecosystems. | Yes—combine with WARM and COLD for ecotoxicity for all of the aquatic species |
| 10. | Wildlife Habitat (WILD) | Uses of water that support terrestrial or wetland ecosystems. | Yes—food chain issues, in addition to WARM, COLD, and EST |
| 11. | Spawning, Reproduction, and/or Early Development (SPWN) | Uses of water that support high quality aquatic habitats suitable for reproduction and early development of fish. | Yes |
| 12. | Shellfish Harvesting (SHELL) | Uses of water that support habitats suitable for the collection of filter-feeding shellfish. | Yes |
| 13. | Hydropower Generation (POW) | Uses of water for hydropower generation. | No—PFAS not expected to impact POW |
| 14. | Industrial Process Supply (PRO) | Uses of water for industrial activities that depend primarily on water quality. | No—as below for IND |
| 15. | Freshwater Replenishment (FRSH) | Uses of water for natural or artificial maintenance of surface water quantity or quality. | No—issues covered under other beneficial uses |
| 16. | Non-contact Water/Secondary Contact Water Recreation (REC-2) | Uses of water for recreational activities involving proximity to water, but where there is generally no body contact with water, nor any likelihood of ingestion of water. | No |
| 17. | Preservation of Biological Habitats of Special Significance (BIOL) | Uses of water that support designated areas or habitats, such as established refuges, parks, sanctuaries, ecological reserves, or Areas of Special Biological Significance, where the preservation or enhancement of natural resources requires special protection. | Not covered individually—covered under WARM, COLD, EST, and WILD |
| 18. | Rare, Threatened, or Endangered Species (RARE) | Uses of water that support aquatic habitats necessary, at least in part, for the survival and successful maintenance of plant or animal species established under state or federal law as rare, threatened, or endangered. | Not covered individually—covered under WARM, COLD, EST, and WILD |
| 19. | Migration of Aquatic Organisms (MIGR) | Uses of water that support habitats necessary for migration or other temporary activities by aquatic organisms, such as anadromous fish. | No—issues already covered under other beneficial uses |
| 20. | Industrial Service Supply (IND) | Uses of water for industrial activities that do not depend primarily on water quality, such as mining and cooling water. | No—concern is the discharge of the water to another location |
| 21. | Navigation (NAV) | Uses of water for shipping, travel, or other transportation by private, military, or commercial vessels. | No—PFAS not expected to impact navigation |
Refining the list of beneficial uses reduces the number of evaluations to the following groupings:
- Aquatic toxicity to both water column and benthic organisms. This beneficial use combines those listed as WARM, COLD, EST, WILD, BIOL, RARE, and SHELL in Table 16-1.
- Protection of human health from ingestion of PFAS when surface water is used as drinking water. Listed as MUN in Table 16-1. The values for this are already covered in Section 8 and in updated tables (the Water and Soil Regulatory and Guidance Values Table Excel file), and discussed further below.
- Protection of human health from exposure to PFAS in the consumption of aquatic organisms, including benthic organisms. In Table 16-1 this encompasses the beneficial uses listed as COMM, AQUA, and SHELL.
- Protection of human health from contact with and ingestion of PFAS in surface water during recreational activities such as swimming and fishing. This beneficial use is listed as REC-1 in Table 16-1.
- Use of the surface water as an agricultural supply (AGR). Use of the surface water in this manner would allow for the uptake of PFAS into plants used for human and animal consumption, stock watering of animals used for human consumption and animal consumption, as well as recharge of excess water to groundwater or other surface water bodies (return water).
- Groundwater recharge is also included, but the issues are the same as those for MUN, AGR, and IND as listed in Table 16-1.
These beneficial use groupings were used to identify the topics to be included in the remainder of this section.
16.1.2 Existing Methods of Protecting Surface Water for Beneficial Uses
This section provides information about the existing methods that regulatory agencies or organizations with jurisdiction use for protecting surface water bodies from discharges of various pollutants. This information provides context for what may be implemented to address potential sources of PFAS in surface water.
After establishing the beneficial uses for a given body of water (lake, stream, creek, estuary, ocean) the regulatory agency or organization implements processes designed to protect those beneficial uses. Typically, this begins with establishing water quality protectiveness standards/criteria or guidance values to protect those specific beneficial uses. As an example, to protect aquatic species, values are established for protection of the health of the fish to allow them to continue to exist in the environment and breed without curtailment. If the fish species is fished for human consumption, then values are also established to allow for fishing to occur without unacceptable effects on those consuming the fish. As values are established for each of the beneficial uses assigned for the water body, the most stringent of the values can be used as the value that is protective of all the designated beneficial uses. In that instance, that value would be used for comparison to other beneficial use values instead of individual values for drinking water and fish protection. For PFAS those values are just beginning to be established.
Once the protective value for the water body has been established, regulatory mechanisms exist to protect the water body to maintain or reduce the concentrations to below the protective values. Discharges to surface water are regulated by state- or federally issued permits. Effluent limits are established in those permits for constituents that could pose a threat to water quality. Establishing appropriate chemical-specific effluent limits protects beneficial uses. The effluent limitations are set so that the concentrations in the surface water body stay below the protective values. In addition to chemical-specific effluent limitations, the permits typically establish acceptable toxicity limitations that must be met in the total effluent. Both effluent and toxicity limitations can take into account mixing with the surface water body within a permitted mixing zone (dilution).
If a water body already has concentrations that are greater than the protective value, then the regulatory agency can establish total maximum daily loads (TMDLs). The TMDLs identify maximum mass discharges for the chemicals that exceed protective values and are used to identify mass loading limits on discharges in the watershed for that water body. Additionally, TMDLs have a component for evaluation of nonpoint sources of discharge within the watershed that include the chemical of concern. If needed, regulations of these nonpoint discharges could be adopted to also limit those discharges. Often, best management practices are established as the control mechanism for nonpoint discharges. If nonpoint discharges are the primary contributor of the pollutant, alternate approaches, such as watershed restoration plans, may be established in lieu of TMDLs. These plans can include best management practices and pollutant minimization components.
During times when a protective value is exceeded and before corrective measures are taken to bring concentrations down below those values, temporary use restrictions can be issued to protect users of the water body. Examples of such restrictions include banning recreation or issuing fish advisories that recommend restricting consumption of various fish species. If the water is being used as a source of drinking water, additional water treatment could be required.
Effluent limitations, TMDLs, watershed restoration plans, and use restrictions have been effective in protecting beneficial uses and reducing risk to receptors for numerous chemicals. It is likely that these measures will also be useful in addressing PFAS.
16.1.3 Status of State and Federal Surface Water Protection Efforts Regarding PFAS
Enforceable vs. Nonenforceable Federal and State Values
Standard – Promulgated values that are enforceable. Example: primary drinking water standard or maximum contaminant level
Criteria – Recommended nonenforceable values that can be used to establish a standard. Example: USEPA water quality criteria
Screening/Guidance Levels – Nonenforceable values that usually represent a de minimus risk and can be used to determine if further action may be necessary. Example: USEPA regional screening levels, action-levels.
At the time of publication, there were no national surface water values for PFAS in the U.S., and only a handful of states have addressed PFAS in surface water; see the Water and Soil Regulatory and Guidance Values Table for updates. Many of the challenges or obstacles to developing surface water criteria are the same as for groundwater, including the large number of individual PFAS, many of which lack toxicity and published physical and chemical properties data. In addition, surface water is used in a greater number of ways than groundwater, representing potential direct exposure routes through dermal contact and water ingestion, and indirect exposure through consumption of fish and shellfish. On October 18, 2021, the USEPA announced its PFAS Strategic Roadmap (USEPA 2021). The Roadmap includes actions that are planned to be undertaken by USEPA. Pursuant to the roadmap, USEPA published draft recommended ambient water quality criteria for the protection of aquatic life in June 2022 for PFOA and PFOS (USEPA 2022). USEPA also issued health advisories for PFOA and PFOS in June 2022 (USEPA 2022, 2022) and surface water quality criteria for the protection of human health are expected fall 2024. In addition, USEPA is increasing the availability of data on PFAS in fish tissue that can be used to finalize the list of PFAS for establishing fish advisories.
At the state level, surface water criteria development has taken a range of approaches. Alaska has adopted health advisory levels for surface water used as drinking water. These levels are used as action levels and are not enforceable under the Clean Water Act. Michigan, Minnesota, and Florida developed their own statewide criteria based on water and fish consumption using state-specific inputs and addressed PFOA and PFOS. Other states are in the process of collecting data or evaluating what approach to take to develop their own criteria in the absence of federal guidelines. Wisconsin is collecting surface water and fish tissue data to support calculation of surface water values. New Hampshire and Vermont have released detailed reports outlining potential strategies and associated costs and timing for developing state criteria. The plan from Vermont describes how its Agency of Natural Resources has developed a framework to establish water quality standards and how it may apply to developing such standards for protection of human health and aquatic life from PFAS. The report concludes that technical challenges and the constraints of deriving water quality criteria (WQC) for PFAS are “logistically difficult, would take a long time, and be very expensive.” It recommends developing fish consumption advisories, tracking USEPA development of aquatic biota criteria for PFAS, incorporating USEPA criteria when they are developed, and continued collaboration with New England states on developing plans for deriving water quality standards (Vermont DEQ 2020). The number of states that have established values for protection of aquatic life is small and includes Michigan and Florida, see the Water and Soil Regulatory and Guidance Values Table for updates to state values.
Surface water criteria are generally established by the states, either by adopting values recommended by USEPA per section 304(a) of the Clean Water Act, or by calculating state-specific criteria that must be approved by USEPA. States have specific responsibilities when setting surface water criteria and submitting that information to USEPA:
- Water bodies must have an appropriate designated use or uses.
- The WQC must support those uses.
- Antidegradation policies to protect high-value waters must be adopted.
- The status of waters must be monitored.
- The standards must be revisited on a regular basis and if a revision is required, the state must obtain USEPA approval.
Most states are still in the process of assessing the extent of PFAS in their surface waters, some only at specific potential source areas.
16.1.4 Survey of States
In the spring of 2020, the ITRC PFAS team sent a survey to the states to gather information on their efforts to address PFAS in surface water. The survey included questions on what media states are monitoring for PFAS, whether PFAS have been detected in surface water, if the states have fish consumption advisories in place for any PFAS, and if the states are contemplating developing surface water quality criteria or guidelines. Other questions addressed the availability of data and information on sampling methods, PFAS-containing foam, and whether states have restrictions to minimize the discharge of PFAS to surface water.
A total of 42 states submitted responses. A summary of key responses is provided in Figures 16-1A-D. These show that of the different media being sampled, 80% of the states are sampling surface water (Figure 16-1A), and of those sampling for various PFAS in surface water, almost 60% detected one or more PFAS (Figure 16-1B). It was determined that 75% of states do not have any criteria, guidance, limits, or standards for PFAS in surface water (Figure 16-1C); however, it is noted that 16% do have some protective measure for surface water that is used as drinking water and 16% have guidance related to fish consumption advisories. Lastly, although 46% of the states were not considering development of criteria for PFAS in surface water, almost 40% felt they needed more information (Figure 16-1d); the remainder are currently developing criteria in response to proposed legislation, legislative mandates, or in response to department-level initiatives. For states that have developed surface water quality values, twice as many states reported using USEPA guidance for developing the values versus those using other procedures and methods.
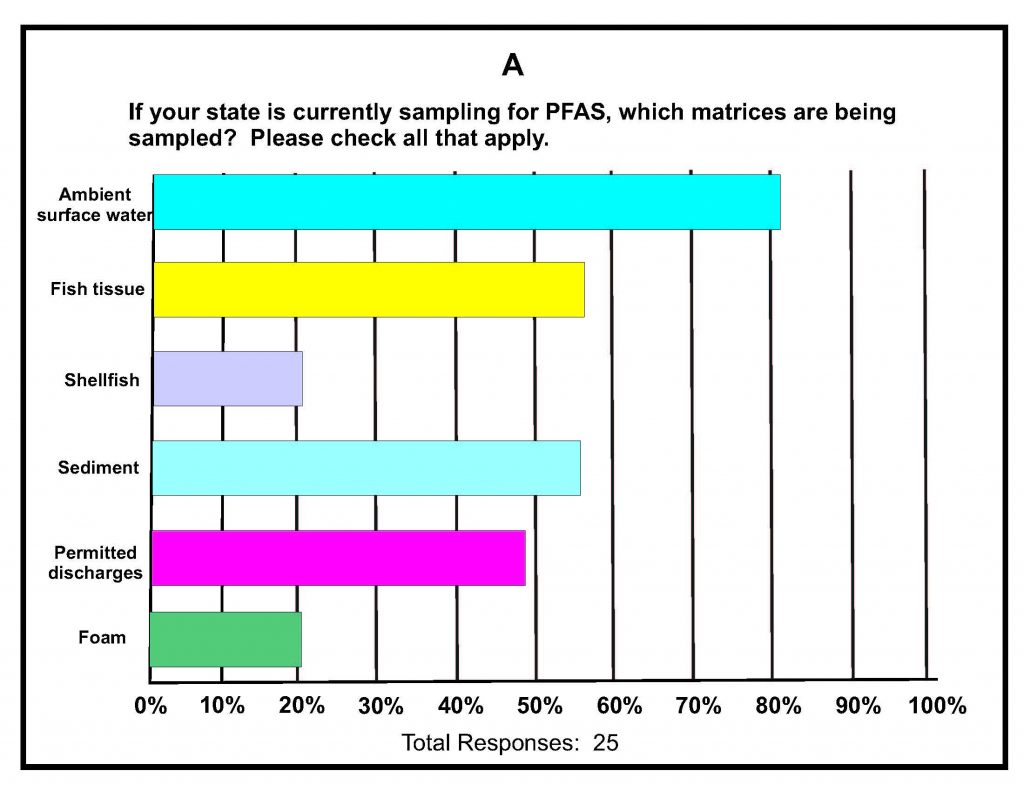
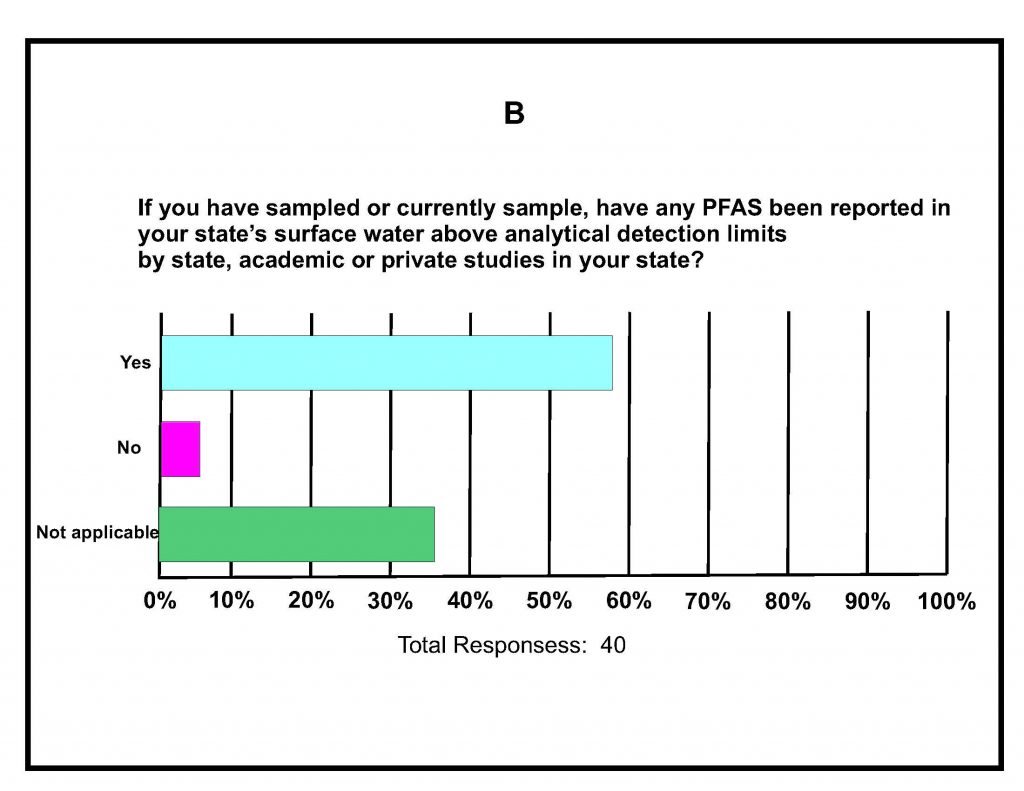
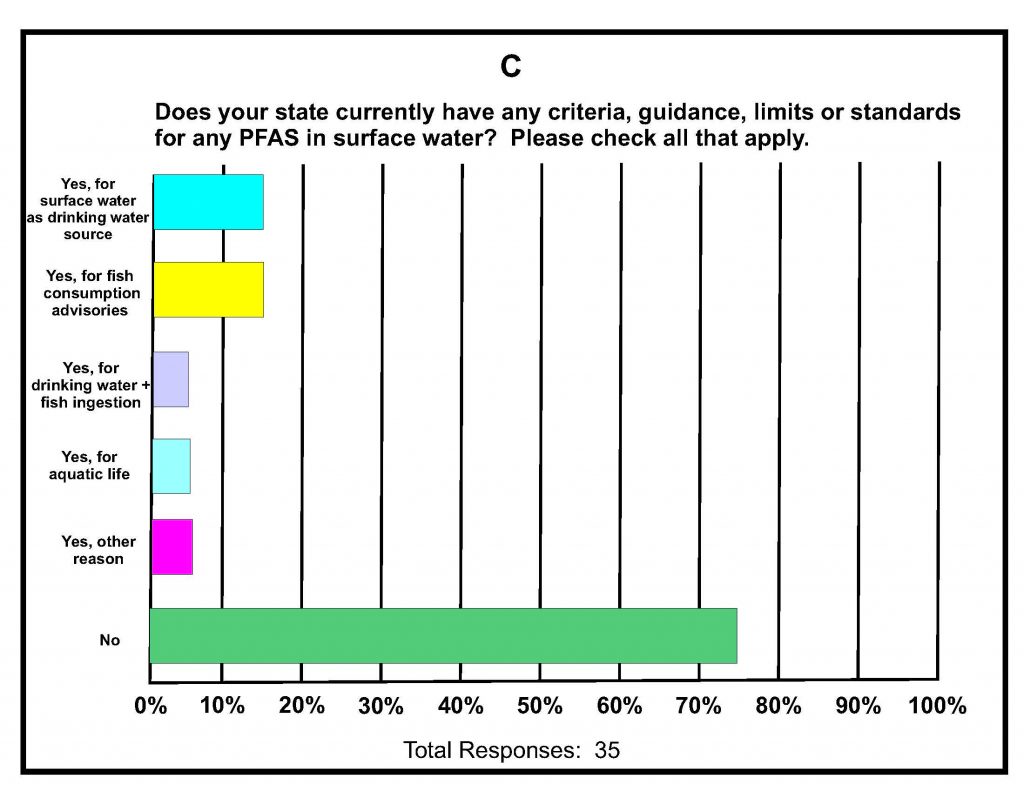
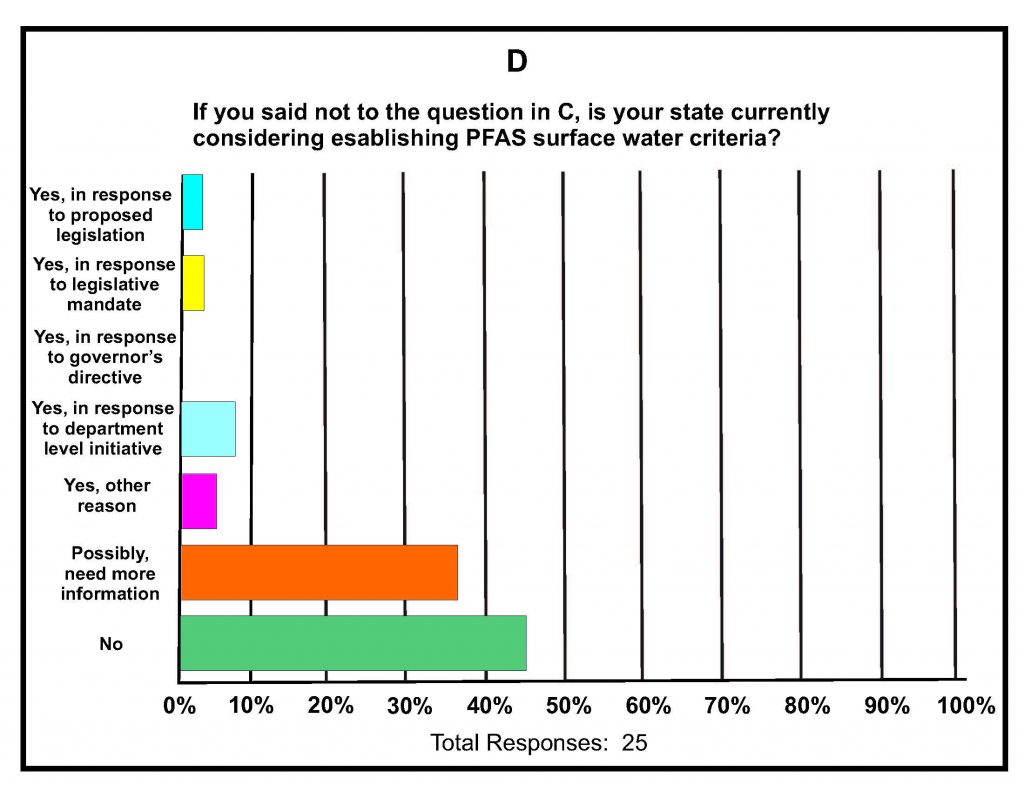
Figure 16-1A–D. Summary of Key Responses from the ITRC State Survey (2020)
Given the rapid pace in the state-of-the-science for PFAS and the desire for PFAS regulations in many states, it is likely there are updates since the time the survey was conducted. However, the PFAS team used the information from the survey to inform what areas to focus on in this surface water quality section and to document the fundamentals for states to consider when developing surface water quality protective values for PFAS. Where relevant, pertinent information from the survey is included in Sections 16.2–16.5.
For updates about states that have developed standards/guidance/limits, see the Water and Soil Regulatory and Guidance Values Table.
16.2 Protection of Human Health
This section discusses the human health aspects of PFAS in surface water. There are other sections in this document that discuss general human health–related PFAS issues in detail that supplement the information in this section. Those sections are Section 7.1, Human Health Effects and Section 9.1, Site Risk Assessment, Human Health.
Under the federal Clean Water Act, states must adopt water quality standards that consist of both designated uses and numerical and/or narrative criteria to protect these uses. As described in Section 16.1, a designated use (also called a “beneficial use” or “designated beneficial use”) in some states is a use of surface waters that is considered desirable and should be protected. As noted in Table 16-1, surface waters have many different beneficial uses, some of which are relevant to human health and some that are not. Uses that are most relevant to human exposure and are considered for most chemicals include municipal and domestic supply (also referred to as drinking water use); agricultural supply (also commonly referred to as irrigation); primary contact recreation; secondary contact recreation/noncontact water recreation; commercial, subsistence, and sport (recreational) fishing (referred to in some states as fish ingestion); and aquaculture (also focused on fish ingestion).
In relation to beneficial uses, the exposure pathways for contaminants in surface water that most warrant consideration of human health for PFAS are included in Table 16-2.
Table 16-2. Exposure Pathways in Relation to Beneficial Uses
| Beneficial Use | Associated Human Exposure Pathway for PFAS |
|---|---|
| Municipal and domestic supply; use as drinking water | Drinking water ingestion and dermal contact during household use; secondary uses may include irrigation for home gardening and produce consumption |
| Commercial, subsistence, and sport (recreational) fishing | Consumption of aquatic organisms (fish and shellfish) that may bioaccumulate PFAS from surface water |
| Primary contact water recreation | Incidental ingestion and dermal contact with water and/or foam during immersion activities such as swimming, waterskiing |
| Non-contact/secondary contact water recreation | Dermal contact with water and/or foam during nonimmersion activities such as wading, boating, fishing; exposures considered to be insignificant for PFAS |
| Agricultural supply | Consumption of crops, dairy products, and meat that may bioaccumulate PFAS from irrigation water; some states may also consider direct contact with irrigation water |
| Aquaculture | Consumption of aquatic organisms (fish and shellfish) that may bioaccumulate PFAS from aquaculture water |
Regulatory agencies use relevant and appropriate use-specific exposure factors combined with chemical-specific toxicity factors (reference doses; cancer slope factors) to develop ambient water quality criteria (AWQC) that are protective of human health for these uses and exposure pathways. The exposure pathways usually considered in development of AWQC are drinking water ingestion (for waters designated for drinking water use) and consumption of aquatic organisms.
At this time, exposure pathways involving ingestion are considered to be the most significant PFAS exposure sources. Recreational uses of surface waters for secondary contact activities, such as wading and boating, which do not involve immersion but may result in dermal contact with water, are considered to be insignificant sources of PFAS exposures. See Sections 17.2.2 and 17.2.3.
Human health criteria for contaminant concentrations in surface water that are protective of fish consumption are also relevant to PFAS exposures. Additionally, some states have developed fish consumption advisories that recommend the frequency of consumption for recreationally caught fish based on levels of contaminants, including PFAS, measured in fish tissue from certain geographic regions or in specific water bodies (Section 16.2.2.6).
The salinity of the surface water body influences the beneficial uses considered and what type of criteria are appropriate (USEPA 2002, p.9). Freshwater criteria apply to waters with salinity less than one part per thousand. Saltwater criteria apply to waters with salinity greater than 10 parts per thousand. The more stringent of freshwater and saltwater criteria apply to waters with salinity between 1-10 parts per thousand. In general, saltwater criteria consider only the consumption of aquatic organisms (fish and shellfish), while freshwater criteria may consider exposure through consumption of aquatic organisms and/or drinking water. The designated uses for freshwater bodies may vary from state to state based on policy and/or actual uses as some water bodies may not support both potable use and aquatic biota consumption. Thus, criteria can be developed for the fish/shellfish consumption pathway alone or for the potable use and fish/shellfish consumption pathways combined. Surface water criteria are usually not developed for potable use alone and generally defer to guidelines such as maximum contaminant levels (MCLs) and maximum contaminant level goals (MCLGs) developed by USEPA and other agencies under drinking water programs. The combined use criterion will be more stringent than the criterion for consumption of organisms only for reasons detailed below.
16.2.1 Input Factors for Development of Surface Water Criteria and Fish Consumption Advisories
Section 16.2.2 includes information and an example equation used to develop human health criteria for relevant exposure pathways. Section 16.2.2.6 provides information and an example equation used to develop fish consumption advisory triggers.
16.2.1.1 Toxicity Values
The development of toxicity values for PFAS is an evolving and dynamic field. Changes in methodology and values, as well as the number of PFAS with toxicity values, should be expected. As a result, the information in this document is current as of March 2023 and the reader is encouraged to consult the most current information at the time of use.
The toxicity values used for both human health criteria and fish consumption advisories are oral reference doses (RfD, ng/kg/day or mg/kg/day) for noncarcinogenic effects and oral cancer slope factors (mg/kg/day)-1 for carcinogenic effects. States may base their WQC and fish consumption advisories on toxicity values recommended by USEPA or toxicity values from sources other than USEPA or develop their own toxicity values (see Section 8.3 for more detail). The toxicity values used for PFAS vary among agencies based on different choices of critical toxicological effect, uncertainty factors, exposure assumptions, and other considerations.
As of early 2023, the USEPA had either proposed or adopted toxicity factors for a limited number of PFAS but had not established surface water criteria for protection of human health. When information becomes available to develop surface water criteria for a PFAS, USEPA may follow a tiered process in selection of toxicity values, as it has done when developing criteria for other contaminants. When USEPA last updated its human health criteria (USEPA 2015), its primary source of updated toxicity values was the Integrated Risk Information System (IRIS), Other sources of toxicity values, for contaminants other than PFAS, reviewed by USEPA in its 2015 update of human health criteria include:
- USEPA Office of Pesticide Programs
- USEPA Office of Pollution Prevention and Toxics
- USEPA Office of Water
- USEPA Office of Land and Emergency Management (for example, Provisional Peer Reviewed Toxicity Values (PPRTVs))
- Agency for Toxic Substances and Disease Registry (ATSDR)
- Health Canada
- California EPA’s Office of Environmental Health Hazard Assessment
The USEPA (2015) updates used a toxicity factor from one of the non-IRIS sources listed above if no IRIS toxicity factor was available, or if the toxicity factor from another source used a newer study or a more current risk assessment approach than IRIS.
USEPA toxicity factors for a limited number of PFAS are at different stages of development and adoption for use in human health risk assessment (See Section 7.1 for information on the health effects of PFAS and Section 9.1 for information on the use of PFAS toxicity values in human health risk assessment). The USEPA’s PFAS Action Plan, which was first developed in 2019 and updated in 2020 (USEPA 2020), states that USEPA will evaluate several PFAS for development of toxicity values. In addition to the reference doses that it developed for its 2016 drinking water health advisories, the USEPA (2014) generated PPRTVs for PFBS. Toxicity factors developed by USEPA’s Office of Research and Development Center for Public Health and the Environment for PFBS (USEPA 2021) and by the USEPA Office of Water for GenX (USEPA 2021) serve as the basis of drinking water health advisories, but these values are currently not listed under IRIS. In late 2022, IRIS published a finalized assessment for PFBA (USEPA 2022) and is in the process of finalizing an assessment of PFHxA (USEPA 2022). Additionally, USEPA scientists are currently developing toxicity values for the following three PFAS(USEPA 2019; USEPA (2023): perfluorodecanoic acid (PFDA) (USEPA 2023), perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS) (USEPA 2023). USEPA Office of Water recently released draft RfDs and oral slope factors for PFOA and PFOS, as well as RfDs for PFHxS and PFNA as a part of their 2023 proposal for National Primary Drinking Water Regulations that have yet to be finalized (USEPA 2023)
16.2.1.2 Protected Populations and Target Risk Levels
The protected populations that are considered in the development of surface water criteria for human health typically include adults. In some cases, other populations, such as lactating women, women of childbearing age, children, and infants, may also be explicitly considered separately, depending on the nature of the chemical and the exposure route. As discussed in Section 16.2, the human health criteria most relevant to PFAS consider ingestion of aquatic organisms (fish and shellfish) and/or drinking water. In general, when calculating criteria for a surface water body that is used for both drinking water and fish consumption purposes, exposure from ingestion of aquatic organisms will likely be greater than exposure from drinking water for PFAS such as PFOS that bioaccumulate in aquatic life when default ingestion rates and bioconcentration factors are assumed.
In the development of human health WQC based on standard exposure assumptions, the relative doses from fish consumption and drinking water are dependent on the bioconcentration factor (BCF) or bioaccumulation factor (BAF), the target population, and their assumed intake rates. The assumed daily dose from fish consumption (22 g/day) is greater than the assumed daily dose from drinking water ingestion (2.4 L/day) for bioaccumulative PFAS such as PFOS (see Sections 16.2.1.3 and 16.2.1.4). However, exposures to bioaccumulative PFAS in drinking water are higher in infants, particularly in those that are breastfed, than in adults, and infants are considered to be a sensitive subpopulation for adverse effects of PFAS (Goeden, Greene, and Jacobus 2019). In contrast to human health criteria for surface water, drinking water guidelines for PFAS developed by USEPA (USEPA 2016, USEPA 2016) and some states are based on exposure assumptions or exposure modeling for sensitive life stages (for example, lactating women, infants (Post 2021)). Minnesota (MPCA 2020) has recently used modeling of early-life exposure in formula-fed and breastfed infants, as well as a higher fish consumption rate specific to women of childbearing age (MPCA 2020), to develop a human health surface water criterion for PFOS; they also developed a human health-based criterion for PFOS in fish tissue.
A target cancer risk level is used along with the cancer slope factor in calculation of criteria based on carcinogenic effects. The selection of the lifetime cancer risk level is a policy choice, not a scientific decision, and the target cancer risk level (for example, 1 in 100,000 or 10-5; 1 in 1,000,000 or 10-6) differs among states, which is one reason why criteria based on cancer risk can vary among different states. Criteria for noncarcinogenic effects are developed such that exposure to the contaminant will not exceed the RfD. When chemicals have the potential to exert both carcinogenic and noncarcinogenic effects (for example, PFOA), the final criterion may be based on the lower of the criteria based on cancer and noncancer effects.
16.2.1.3 Exposure Factors
The exposure factors typically considered in development of criteria for ingestion of drinking water and/or aquatic organisms include assumed body weight, drinking water and/or aquatic organism (fish/shellfish) consumption rates, and relative source contribution (RSC). These exposure factors and their use in developing criteria are described below.
As previously stated, the default adult body weight is usually used when developing human health criteria. USEPA currently recommends a default adult body weight of 80 kg for development of human health criteria (USEPA 2015). However, some states’ criteria are based on the older recommended value of 70 kg. Alternative body weight assumptions for specific ages, sexes, or other subgroups can be found in the USEPA Exposure Factors Handbook (USEPA 2011), and certain states may use these values.
States typically use default ingestion rates recommended by the USEPA for the specified environmental media, for example, drinking water or fish tissue; however, some states use state-specific values, especially for fish ingestion. The adult drinking water consumption rate is usually used for human health criteria. In 2015, USEPA updated its recommended default adult drinking water consumption rate for human health criteria from 2 L/day to 2.4 L/day (USEPA 2015). It is noted that the relevant body weight-normalized exposure parameter (L/kg/day) is 0.03 L/kg/day with the updated body weight and ingestion volume, which represents a very small change from 0.029 L/kg/day based on the older values. Similarly, USEPA also updated the default rate of 22 g/day for fish consumption from the previous value of 17.5 g/day (USEPA 2015). Some states consider state- or region-specific rates that have been developed based on consumption data from their region, including higher consumption rates by tribes and/or for subsistence fishing in some cases (USEPA 2014).
Under current USEPA (2000) guidance, an RSC is used when a human health criterion is based on an RfD (noncancer effects), and USEPA used an RSC (USEPA 2015) in its updates of noncarcinogenic criteria. The RSC accounts for potential non-drinking water exposures to chemicals and is used in the development of health-based guidance and standards developed by the USEPA and related state programs. Conceptually, the RSC is the percent of total exposure assumed to come from exposure arising from surface water (ingestion of water and aquatic organisms, or ingestion of organisms only) at the criterion concentration (for example, an assumed RSC of 20% for a drinking water criterion means that the target population is assumed to be exposed to 80% of the RfD from non-drinking water sources). It is intended to ensure that total exposure from all sources (surface water and non-surface water–related) does not exceed the RfD. USEPA guidance specifies an RSC of 20–80 percent, with a default of 20 percent (the most stringent possible value) when data to derive a chemical-specific value are not available (USEPA 2015). Some states and USEPA use the 20 percent default value in their PFAS drinking water guidelines, while other states (for example, MN, NH, NY) use higher RSC values based on estimates of non-drinking water exposures from human biomonitoring data or in consideration of certain life stages.
16.2.1.4 Bioaccumulation, Bioconcentration, and Biomagnification Factors
Water to fish transfer factors are useful in the development of water quality criteria and to inform risk-based evaluations. Three types of factors can be used by risk assessors to relate environmental concentrations of a chemical (for example, in water, sediment, soil, or prey) to concentrations within certain organisms. These factors include bioaccumulation factors (BAF), bioconcentration factors (BCFs), biomagnification factors (BMFs) and associated trophic magnification factors, each of which are defined in the text box below, and detailed in Section 5.5. These factors are frequently used for risk assessment of biota such as fish and shellfish consumption by human fish consumers, as well as wildlife. The USEPA has outlined methods for developing BAFs, which are recommended for risk assessment of most chemicals, whereas BCFs and BMFs typically provide useful information about the fate, transport, and ecological risks of chemicals (USEPA 2003).
Transfer Factors
Bioconcentration factors (BCF, L/kg) represent the direct uptake of PFAS by an organism from the water column (through the gills) and are measured as the ratio of the concentration in an organism to the concentration in water. Typically derived from laboratory studies.
Bioaccumulation factors (BAF, L/kg) represent the amount of PFAS taken up from bioconcentration plus the contribution of PFAS in the diet of that organism. Typically estimated from field studies.
Biomagnification factors (BMF; typically unitless) describe the increase in tissue concentration as one moves up the food chain based on a predator/prey relationship (always measured in the field); often defined as the concentration of chemical in an organism divided by the concentration of chemical in its food.
Trophic magnification factors (TMF; typically unitless) express the change in contaminant level per trophic level, and therefore describe the biomagnification between different trophic levels of the food web.
Certain PFAS are highly bioaccumulative in aquatic organisms such as fish, and this is especially true for long-chain PFAS such as PFOS. For the risk assessment of bioaccumulative and bioconcentrating chemicals such as PFAS, BAFs or BCFs should represent the tissues that are consumed by humans (for example, fillets or muscle tissue) and should also represent the trophic level of the fish species of interest (for example, secondary consumer or top-level predatory fish). Both BAFs and BCFs are specific to the chemical and organism in which it is detected. For BCFs, BAFs, or BMFs, larger values indicate greater accumulation in organisms, which in turn result in lower surface water criteria. At this time BCFs, BAFs, and BMFs for PFAS are primarily based on measured data. Examples of these values for PFAS in aquatic organisms are available in Table 5-1 (as a separate Excel file) and discussed in Section 5.5.
When the standard USEPA equation shown in the text box in Section 16.2.2 is used with standard exposure assumptions (drinking water ingestion – 2.4 L/day; fish consumption – 22 g/day) to develop human health criteria, the assumed contribution to total exposure from fish consumption is greater than from drinking water when the BCF or BAF is >110. As the numerical value of the BCF or BAF increases, the proportion of assumed exposure from fish consumption continues to increase. Given the mathematical impact of BAFs, BCFs, and BMFs on resulting WQC, there are several considerations for data use and applicability. One example of these considerations is the applicability of values estimated from studies on nonnative species or from water bodies with different water chemistry from the sites intended for protection. The use of central tendency versus upper percentile values may result in less stringent criteria but may be less appropriate in cases where BAFs for a given compound vary by orders of magnitude. The USEPA encourages the use of site-specific modification to BAF, BCF, and BMF selection where such decisions are appropriate and scientifically defensible (USEPA 2000).
16.2.2 Human Health Criteria Development for Beneficial Uses of Surface Water
As shown below, USEPA’s standard equation for development of surface water criteria incorporates terms related to receptor characteristics, exposure, and toxicity values, as described in earlier sections.
Standard USEPA Equation for Combined Drinking Water + Fish/Shellfish Consumption
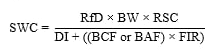
SWC = surface water criterion (mg/L)
RfD = reference dose (mg/kg/day)
BW = body weight (kg)
RSC = relative source contribution (unitless)
BCF = bioconcentration factor (L/kg)
BAF = bioaccumulation factor (L/kg)
FIR = fish ingestion rate (kg/day)
DI = drinking water ingestion rate (L/day)
It is noted that the USEPA (2015) updated human health ambient water quality criteria describe a similar but more complex equation incorporating trophic level–specific fish consumption rates and BAFs (or BCFs) when available. However, this approach does not appear to be applicable to PFAS since current evidence indicates that BAFs for PFAS are not clearly associated with trophic level, and trophic level–specific BAFs have not been developed for PFAS.
This section outlines issues that are relevant to development of surface water criteria for specific beneficial uses. These criteria are based on the protection of human health from exposure via intentional ingestion of contaminated media, including:
- drinking water use, 16.2.2.1
- subsistence, commercial, and sport fishing, 16.2.2.2
- drinking water combined with subsistence, commercial, and sport fishing, 16.2.2.3
- contact recreation (incidental ingestion of and skin contact with surface water, sediment, and PFAS-containing foam), 16.2.2.4
- use for agriculture, 16.2.2.5
- fish consumption advisories, 16.2.2.6.
Using USEPA methodology, the surface water criteria described in Sections 16.2.2.1, 16.2.2.2, and 16.2.2.3 can be derived using a variant of the equation in the text box to the right. As described in Sections 16.2.2.1 and 16.2.2.2, terms accounting for exposure from drinking water or fish consumption can be removed as appropriate for the designated use of the water body.
The Environmental Council of the States (ECOS) published their updated white paper, Processes and Considerations for Setting State PFAS Standards in March 2023 (ECOS 2023).
16.2.2.1 Waters Designated for Domestic/Municipal Supply (Drinking Water Use)
As noted earlier, surface waters that are designated only for drinking water use may use drinking water guidelines already developed by USEPA or state agencies as WQC. Current state and federal drinking water guidelines for PFAS are found in Water and Soil Regulatory and Guidance Values Table. How certain states apply this may depend on certain regulatory considerations and authorities (ECOS 2023). Some states derive this type of surface water criterion using the equation above but omit the denominator terms for bioaccumulation/bioconcentration factors and fish ingestion rates. This equation determines a drinking water value based on human health risk assessment but does not include consideration of analytical or treatment removal factors. Additional discussion of drinking water guidelines may be found in Section 8.3.
Numerically, surface water quality criteria for the protection of drinking water may be the same as or differ from drinking water standards such as MCLs, which apply to public water systems, or ambient groundwater quality standards. Drinking water standards such as MCLs are often higher than the strictly health-based goals derived by the previously described equation because they consider analytical and treatment limitations, which consider higher values. In contrast, surface water criteria do not consider these factors. However, almost all the current state and federal drinking water guidelines for PFAS are set at health-based goals because analytical and/or treatment removal considerations are not included as limiting factors in the development of the guidelines. Some states may have the authority to adopt existing drinking water values, such as MCLs, as surface water quality criteria for the protection of drinking water sources. This varies due to states’ regulatory authorities and definitions of surface waters considered acceptable for potable use. Alternatively, some states may derive criteria that differ from drinking water guidelines due to consideration of drinking water use combined with other designated uses such fish/shellfish consumption.
16.2.2.2 Waters Designated for Subsistence, Commercial, and Sport (Recreational) Fishing (Consumption of Aquatic Organisms [Fish and Shellfish] Only)
Surface water criteria for fish and shellfish consumption rely on assumptions about consumption rates as well as the relationship between chemical concentrations in water and the resulting tissue concentrations in consumed aquatic organisms.
Default fish consumption rates recommended by USEPA and choices made by states to reflect regional consumption patterns were discussed in Section 16.2.1.3. The quantifiable relationship between chemical concentrations in water and the concentrations in tissue is represented by transfer factors such as BCFs, BAFs, or BMFs, which are defined in Section 5.5 and included in the text box in Section 16.2.1.4.
The equation used to derive this type of criterion, using USEPA methods, would be the equation in the box above but omitting the drinking water ingestion rate. This equation relies on toxicity values (Section 16.2.1.1) and exposure factors (Section 16.2.1.3), as well as BAFs or BCFs for the specified chemical. More stringent toxicity values, higher exposure factors (for example, fish consumption rates), or larger BAFs or BCFs result in lower surface water criteria. For example, criteria based on the higher fish intake rates for subsistence fish consumers would be more stringent than criteria based on the average intake rates for U.S. consumers or sport fishers. Depending on state level or regional policies, such criteria may be developed separately for sport (recreational) and subsistence fishing practices. Aquaculture operations may be required to meet similar criteria for rearing, harvesting, or selling fish depending on the regulatory authority of certain states. This type of surface water criteria is typically developed in consideration of fish, especially predatory fish, as these are more commonly caught and consumed by the public than are shellfish. However, similar criteria can be developed for shellfish (for example, bivalves and crustaceans) using similar risk assessment methods.
16.2.2.3 Waters Designated for Combined Use as Drinking Water and for Subsistence, Commercial, and Sport (Recreational) Fishing
Freshwaters may support and/or be designated for both drinking water and fish consumption beneficial uses. This requires consideration of factors discussed in Sections 16.2.2.1 and 16.2.2.2. The full equation in the box above provides the USEPA method for deriving this type of criteria. Default input values would be similar to those listed for each use separately. Surface water criteria established for both drinking water and fish consumption will be more stringent than criteria for either use alone.
16.2.2.4 Waters Designated for Contact Recreation
WQC for recreational contact, such as swimming and wading, consider exposure primarily due to incidental ingestion and dermal contact with surface water, sediments, and potentially PFAS-containing foams. There is no standardized equation for development of criteria protective of exposure to recreational water, but certain equations in the USEPA’s Risk Assessment Guidance for Superfund (RAGS) provide a framework for developing screening levels for recreational contact (USEPA 2004). Specifically, equations for estimating chemical intake by incidental ingestion of water and dermal absorption from water during swimming or wading are available in Chapter 3 of the RAGS Part E, Supplemental Guidance for Dermal Risk Assessment (USEPA 2004). There are several gaps in the current literature that limit the application of these equations, including scant information on skin permeability of several PFAS and how distribution in the water column influences dermal contact and potential for exposure to aerosols. Knowledge regarding volatilization and inhalation exposure is still evolving at this time.. Currently available information on partitioning of PFAS to air and the air/water interface is reviewed in Sections 5.2.4 and 5.2.4.1.
Dissolved-Phase PFAS
At this time, primary or secondary contact recreation with surface water is not expected to be a significant pathway for human exposures to PFCAs and PFSAs (for example, PFOS) as compared to drinking water or to consumption of aquatic organisms. Current evidence suggests that PFAS are poorly absorbed through the skin (discussed in Section 17.3.1.2). Studies performed by some state agencies estimated the risks associated with observed PFAS concentrations in water via recreational exposures such as swimming, and concluded that the risks were low (MDHHS 2019, MDH 2019, Vermont DEQ 2020, MPCA 2020). Recreational screening levels are likely to be orders of magnitude higher than concentrations that are protective of consumption fish/shellfish, drinking water use, or some estimates of toxicity toward aquatic wildlife. However, there is uncertainty regarding dermal absorption capabilities (for example, skin permeability coefficients) of most PFAS, both short- and long-chain, that would have a significant impact on derived guidance values. One additional area of concern may come with inhalation in an environment where water-to-air transfer may occur from aerosol-borne PFAS in sea sprays or dam mist near known source areas (Johansson et al. 2019). Knowledge in these areas and knowledge regarding the volatilization and inhalation potential of PFAS is very preliminary, still evolving, and subject to change (see Section 5.3).
PFAS in Foams
In contrast to dissolved-phase PFAS, PFAS-containing foam may occur at and above the air-water interface on surface waters (see Section 16.5 for details). However, as noted in Section 16.5, the presence of foam does not necessarily indicate the presence of PFAS. That said, observations concerning PFAS-containing foams present in surface waters have been reported in several states and present concerns for exposure from prolonged skin contact or incidental ingestion by small children. Unlike PFAS dissolved in water, foams may remain on the skin for a longer period, which may elicit public concerns. There are currently no WQC for the formation of foams, but some states may consider developing contact standards for exposure to foams. As noted above, a critical parameter in developing risk-based screening levels for recreational contact is the skin permeability coefficient, which is a measure of dermal absorption. There is currently high uncertainty and limited information available regarding skin permeability for PFAS (see Section 17.3.1.2), and this lack of data poses major challenges to risk assessment for human dermal contact with PFAS. It is anticipated that exposure to PFAS-containing foam on surface waters poses a significantly lower risk to human receptors than direct ingestion from food and water, but if combined with drinking water or occupational exposure, could pose an added toxicological burden.
Using a similar approach to that described above, along with USEPA guidance (USEPA 2000, 2011, 1989), some states may decide to develop surface water criteria for PFAS-related foams. However, these surface water criteria would likely be far higher than any criteria needed to protect surface water for other uses by humans and wildlife and would be applicable to a limited number of sites. Some states, such as Minnesota, have published qualitative guidelines (MPCA 2020). In the future, some states may develop human health-based criteria for PFAS-containing foams in surface waters that would be protective of recreational exposures such as swimming and wading. It is also challenging to sample foam in surface waters, as described in Section 16.5.
16.2.2.5 Waters Designated for Agricultural Supply
One of the beneficial uses of fresh water is agricultural supply, for irrigation of crops for direct human consumption and silage for dairy or beef livestock that may ultimately lead to indirect human consumption (milk or beef ingestion). Since PFAS are known to bioaccumulate and have been detected in edible crops, produce, dairy, and meats (Section 5.6) (ATSDR 2018; USFDA 2019), there are potential concerns regarding acceptable levels of PFAS in surface water that will be protective of human health if used for irrigation of crops and silage.
The rapidly expanding literature about PFAS uptake into crops is useful in identifying a few general trends and is discussed in depth in Sections 5.2.3 and 5.6. The potential for PFAS bioaccumulation in plants ranges from low (0.1) to approximately 10 times the soil concentration in many studies, although plants with high water content (for example, lettuce) could exhibit considerably higher bioaccumulation of certain PFAS (see Table 5-2, provided as a separate Excel file and discussed in Section 5.6.2). Chain length is a significant factor in availability for uptake into plants. While both longer chain and shorter chain PFCAs and PFSAs may be taken up by plant roots, there is generally greater translocation and distribution of shorter chain PFAS into the remainder of the plant, including the aboveground tissues (Section 5.6.2). In general, there is greater accumulation in vegetative tissues (for example, leaves and stems) than in storage tissues such as fruits and seeds (Section 5.6.2).
There is currently no USEPA guidance for development of surface water criteria for irrigation and livestock watering. However, approaches have been developed by some states, and other countries such as Canada (ECCC 2017), that consider human dermal and inhalation exposure to irrigation water and consumption of irrigated produce (for example, University of Florida (2018)). These references may be consulted for additional information on irrigation and livestock protection.
16.2.2.6 Fish Consumption Advisories Based on Consumption Frequency
Fish consumption advisories are health-protective recommendations developed by states for frequency of consumption of recreationally caught fish meals. These advisories may be applicable statewide, in certain regions of a state, or to specific water bodies or reaches/segments of water bodies. Fish consumption advisories are not surface water criteria and are not regulatory in nature. The advisories may be issued for the general public, and they may be more stringent for specific groups of people at higher risk, such as women of childbearing age, pregnant or nursing women, or children. At the time of publication of this document, several states (for example, AL, CT MI, MN, NJ, WI) have issued fish consumption advisories for PFAS, particularly PFOS.
Standard USEPA Derivation of Fish/Shellfish Tissue Trigger Concentrations Used in Development of Consumption Advisories

Where:
DTC = trigger concentration for daily consumption (µg/g)
BW = assumed human body weight (kg)
RfD = chronic oral reference dose (µg/kg/day)
MS = meal size (g/day)
Trigger concentrations for daily consumption are based on calculation of the concentration of a contaminant in fish tissue that results in exposure equal to the RfD from a fish meal. Trigger concentrations for less frequent meal consumption are calculated by multiplying trigger concentration for daily consumption by appropriate factor (for example, daily – 7; monthly – 30).
Advisories are developed by comparing fish tissue concentrations in wet weight (“triggers”) that do not result in unacceptable risks for consumption of meals at different frequencies (for example, unlimited [daily]; once per week, once per month, once per year) with concentrations of the contaminant measured in fish tissue. The advisories may be species- and water body–specific, based on data for PFAS tissue concentrations from the species in the water body, or they may apply regionally and/or to multiple species. Typically, limits are developed for muscle fillets of commonly caught and consumed fish, often accompanied by a recommendation to avoid consumption of skin, fat, and other nonmuscle parts of the fish. If there are populations whose consumption patterns exceed the assumptions of the advisory (for example, consumption of whole fish), there may be a potential for insufficient protection.
Assumptions and parameters used in the calculation of fish tissue trigger levels include:
- the reference dose for each PFAS
- a consumer’s body weight (BW)
- the size of the fish meal
The generalized equation for development of fish tissue trigger concentrations based on noncancer effects used for fish consumption advisories are shown in the text box; a different equation (not shown) would be used for advisories based on cancer risk.
16.3 Protection of Biota
16.3.1 Overview and Purpose
This section presents the technical methods and information needed to develop or review surface water criteria for PFAS that would be protective of aquatic life and their uses. There are other sections in this document where general ecological issues associated with PFAS are discussed in detail, and that information supplements the information contained in this section. That additional information can be found in Section 7.2, Ecological Toxicology, and Section 9.2, Ecological Risk Assessment. Section 16.3.2 provides information regarding ecotoxicological data for assessing water quality criteria for PFAS that were available during development of previous versions of this document and still provides a starting point for evaluation of available values. The section is not intended to present an exhaustive compilation of the currently available data as this is an active field of research in which new information is regularly provided. It is recommended that the reader search for updated ecotoxicological data prior to development of water quality criteria for the protection of wildlife.
The types of aquatic life to be protected are usually defined by the various beneficial uses related to surface water that are described in Section 16.1.1. USEPA guidance (USEPA 1985) for derivation of such criteria are primarily intended to protect all but the most sensitive aquatic organisms from exposure to chemicals in surface water or sediment porewater. Considerations also exist to derive criteria for protection of aquatic-dependent avian and mammalian wildlife via calculation of a “final residue value” that can factor into final selection of the chronic criterion (USEPA 1985), or a more explicit “wildlife criterion” using methods presented for the Great Lakes Initiative (GLI; (USEPA 1995)).
Aquatic life criteria share the levels of protection afforded by the Clean Water Act and USEPA guidelines (USEPA 1985) in that criteria are derived using toxicity tests with aquatic organisms in which survival, growth, and reproduction are measured. These data are compiled to derive criteria intended to protect against unacceptable adverse effects to most animal taxa in the aquatic community, which is most commonly calculated to represent protection of approximately the 95th percentile of tested aquatic genera. As a result, acute and chronic criteria concentrations are generally said to represent protection of all but 5% of the most sensitive aquatic species. Criteria can also be lowered to protect particularly important species such as recreationally or economically important species or listed threatened or endangered species.
The following sections summarize the general USEPA methods available for derivation of aquatic life criteria for PFAS, with a focus on how to select the most appropriate toxicity test endpoints related to USEPA guidance (USEPA 1985). Available ecotoxicity data are then summarized for all freshwater and marine aquatic species relevant for derivation of aquatic life criteria, including invertebrates, vertebrates, and algae/vascular plants. This section closes with a summary of information necessary to develop criteria to protect aquatic-dependent wildlife such as birds or mammals, including permissible tissue PFAS concentrations, bioaccumulation and bioconcentration factors, and other food chain effects.
16.3.1.1 Derivation of Aquatic Life Protection Criteria—Methods Summary
The general approach for derivation of aquatic life criteria, according to USEPA guidance (USEPA 1985), is briefly summarized below, along with the definition of key terms. The first step is to compile acute and chronic toxicity data that meet the USEPA (1985) guidelines for the relevance and reliability of each study. This evaluation for scientific relevance and reliability largely focuses on test duration, survival in the control treatment, and methods, with distinctions made between acute (short-term) or chronic (long-term) studies. For example, acute toxicity studies must have an exposure duration of 96 hours (although 48 hours is acceptable for more short-lived species, such as cladocerans and midges), organisms must not be fed during the study, and the endpoint must be mortality, immobilization, or a combination of the two. Chronic toxicity studies must be conducted using exposure durations that encompass the full life cycle or, for fish, early life stage and partial life cycle studies. The acceptable endpoints for chronic tests include survival, growth, and reproduction (see Section 16.3.1.4). The duration of chronic studies may be many days, weeks, or months.
To develop criteria that are protective of the diverse array of aquatic biota, an extensive database representing multiple test species, genera, and taxa is required. A minimum database of acceptable studies representing at least eight specific taxonomic families of aquatic organisms is also required. This is done to ensure that criteria are derived based on data that represent the widest possible range of likely sensitivities encountered in the environment. These minimum database requirements differ for freshwater versus saltwater species as presented in Table 16-3.
Table 16-3. Minimum database requirements for derivation of aquatic life criteria (USEPA 1985)
| Freshwater | Saltwater |
|---|---|
| A bony fish in the family Salmonidae | Two families in the phylum Chordata |
| A second family of bony fish (preferably a commercially or recreationally important warm water fish) | A family in a phylum other than Arthropoda or Chordata |
| A third family in the phylum Chordata | Either the Mysidae or Penaeidae family |
| A planktonic crustacean | Three other families not in the phylum Chordata (may include Mysidae or Penaeidae, whichever was not used above) |
| A benthic crustacean | Any other family |
| An insect | |
| A family in a phylum other than Arthropoda or Chordata | |
| A family in any order of insect or any phylum not already represented |
For each species with acceptable acute toxicity data, the species mean acute value (SMAV) is calculated as the geometric mean of available 48- to 96-hr median lethal concentrations (LC50s) or median effect concentrations (EC50s) for each species. The genus mean acute value (GMAV) is then calculated as the geometric mean of available SMAVs for each genus. The lowest 5th percentile of the distribution of available GMAVs is identified as the final acute value (FAV), which is divided by two to determine the criterion maximum concentration, which is more commonly termed the “acute criterion.” The criterion continuous concentration, or “chronic criterion” can either be calculated using the same 5th percentile calculation as the FAV if all eight minimum database requirements are met, or if they are not met by dividing the FAV by the ratio of acute to chronic effects, termed the acute to chronic ratio (ACR). The ACR is determined from those species with both acute and chronic data available usually as a geometric mean of the available species ACRs. ACR values are one of the factors used to derive chronic surface water criteria for chemicals (USEPA 2008). They may also be used in the development of aquatic life screening values when acute toxicity data may be available but not enough chronic toxicity data are available for a chemical. Generally accepted ACR values have not yet been developed for PFAS and would need to take into account the diversity of chemical structures and behavior of PFAS. See the discussion in Section 16.3.2 regarding the availability of acute and chronic data for select PFAS.
16.3.1.2 Problem Formulation
Recently, USEPA has been developing ambient water quality criteria documents following an ecological risk assessment framework (USEPA 1992) to provide a logical approach to criteria derivation based on the chemical’s characteristics, fate and transport, and mode of toxic action. This information for PFAS is summarized below to assist with the development of a conceptual model and identification of endpoints needed for states to derive aquatic life criteria.
16.3.1.3 Conceptual Model
A conceptual model consists of a written and/or graphical representation of the linkages between the exposure characteristics of the chemical and the ecological endpoints of relevance to criteria derivation. PFAS will be transported to surface waters from direct soil runoff, groundwater discharge, atmospheric deposition, or point source discharges (see Figure 17-1). Once transported into surface waters, PFAS exposures to aquatic organisms will occur via two main pathways: direct from water (bioconcentration) and via the organism’s diet as well as water (bioaccumulation). These pathways are also described and quantified in Section 5.5.
Biomagnification is also an important factor for some PFAS (for example, those with carbon chain lengths of eight or more) (Section 5.5). Section 16.3.3 describes procedures to evaluate uptake of PFAS in aquatic-dependent wildlife.
This simple conceptual model suggests that aquatic life criteria for PFAS will need to consist of two different kinds of toxicological information:
- Toxicity data generated from water-only exposures
- Toxicity data with effects measured on the basis of receptor tissue concentration and diet, from which bioaccumulation and bioconcentration factors are estimated to back-calculate to a protective PFAS concentration in water (see Section 16.3.3.3).
Toxicity data measured on the basis of receptor tissue concentration can also be used to derive purely tissue-based aquatic life protection criteria for PFAS. Tissue-based aquatic life criteria would be subject to less uncertainty because the effects levels directly relate toxic dose and exposure pathways, and because BAFs or BCFs would not be needed to back-calculate to a water concentration. This approach has recently been used for methylmercury (USEPA 2010) and selenium (USEPA 2016) in fish in which tissue-based criteria concentrations take precedence over water column-based concentrations.
16.3.1.4 Assessment Endpoints
Assessment endpoints represent the expression of environmental values to be protected by the management framework (USEPA 1992). In the case of ambient water quality criteria, the values to be protected are aquatic life and their uses. As discussed in Section 16.3.1, the levels and types of protection are those afforded by the Clean Water Act and USEPA guidelines (USEPA 1985). These guidelines specify protection of all but 5% of the most sensitive aquatic organisms with respect to survival, growth, and reproduction.
16.3.1.5 Measurement Endpoints
Measurement endpoints represent the direct empirical measurements of chemical exposure and biological effects that are used to ultimately represent the assessment endpoint (USEPA 1992). For PFAS, the relevant measurement endpoints are as follows:
- Measures of exposure: Given the conceptual model noted above, PFAS measurements would need to include both direct aqueous concentrations and aqueous concentrations estimated using appropriate bioaccumulation or bioconcentration factors (see Section 16.3.3 and Section 9.2.2). Sufficient single chemical toxicity data for aquatic life criteria mainly exist for only PFOA and PFOS, although Tier II methods have been used by Giesy et al. (2010) for PFBS and by Divine et al. (2020) for acute and chronic values for 21 PFAS. Mixture studies for commonly detected PFAS are limited with no consensus on additivity, synergism, or antagonism. Thus, it is difficult to determine at this time whether measures of exposure could be considered additive for either PFOA + PFOS, or even for other PFAS.
- Measures of effect: According to USEPA guidance (USEPA 1985), measures of effect (either acute or chronic) are limited to those representing survival, growth, and reproduction. Therefore, only PFAS toxicity endpoints that either directly measure, or can directly represent, these endpoints should be used for derivation of aquatic life criteria. According to toxicity data summarized in Section 16.3.2 below (and in Section 7.2), available PFAS toxicity endpoints include several sublethal endpoints that could potentially be used to represent growth or reproduction. These include endpoints such as development, percent emergence, time to metamorphosis, and development of malformations. Therefore, states will need to determine whether or not these sublethal effects can be considered reliable quantitative measures of chronic effects of relevance to development of criteria. USEPA developed some logical considerations for this determination relative to endocrine-disrupting chemicals. These considerations may be applicable to PFAS (USEPA 2008).
16.3.2 Availability of Ecotoxicological Data
Toxicity data for establishing surface water quality criteria are available in peer-reviewed sources, mainly the primary literature. As noted in Section 7.2, most aquatic toxicity data are for PFOS, PFOA, and several other PFAS, including PFNA, PFBA, and PFBS. USEPA has curated peer-reviewed sources of ecotoxicity data for PFAS into the USEPA Ecotoxicology (ECOTOX) Knowledgebase (USEPA 2023). In addition, as stated in Section 16.1, USEPA has published draft national recommended aquatic life criteria for PFOA and PFOS in freshwater for public comment (USEPA 2022, 2022) and a fact sheet for the criteria (USEPA 2022). Furthermore, USEPA has published their responses to external peer reviews of the draft criteria (USEPA 2022, 2022).
This section provides a high-level overview of the available ecotoxicity data retrieved at the time and does not represent an exhaustive literature review for the classes of organisms specifically required for developing aquatic life surface water quality criteria. As noted in Section 7.2, the ecological toxicology of PFAS is an active area of research and users are encouraged to query the literature for updated values. Furthermore, states that undertake criteria development should review the studies discussed here and others that are subsequently published to determine if they meet regulatory requirements prior to using them for WQC development.
When developing surface water quality criteria protective of aquatic receptors, several criteria should be met when selecting appropriate studies, such as inclusion and adequate control animal responses and availability of details on experimental design. Test animals should be native and have reproducing wild populations. Also, note that many published endpoints are based on nominal rather than measured PFAS exposures; where measured concentrations are used, they can range from very low to very high percentages of the nominal values (<10–240%). Thus, it is recommended to use measured concentrations for establishing criteria.
The following sections highlight available data, or lack thereof, for the various taxonomic families required for WQC development (refer to Table 16-3 and Section 16.3.1.1).
16.3.2.1 Bony Fish
Aquatic criteria development requires toxicity data for a salmonid species and one other species of bony fish. Fish toxicity studies are mainly focused on PFOS, for which data for multiple bony fish species (for example, rainbow trout (Oncorhynchus mykiss), sheepshead minnow (Cyprinodon variegatus), fathead minnow (Pimephales promelas), and zebrafish (Danio rerio) are available. As discussed in Section 5.5, PFOS preferentially accumulates in fish tissue relative to other PFAS. Overall, the data for other PFAS are generally limited to a single species or are lacking entirely (see Section 7.2).
16.3.2.2 Salmonids
Acute salmonid studies on the rainbow trout (O. mykiss) have been conducted under both fresh- and saltwater conditions for PFOS. EC50 range from 17 mg/L to 22 mg/L (Robertson 1986; Palmer, Van Hoven, and Krueger 2002); a no observed effect concentration (NOEC) was reported at 6 mg/L (Palmer, Van Hoven, and Krueger 2002). No chronic studies were identified for this or other salmonid species.
16.3.2.3 Other Fish Species
Several acute and chronic studies are available for non-salmonid species (see Section 7.2) and for PFAS other than PFOS. Data from these studies indicate acute toxicity of PFOS on the same order of magnitude as that observed for the rainbow trout, with some species perhaps even more sensitive; LC50s for zebrafish (D. rerio; a freshwater native to Asia) and fathead minnow (P. promelas, a freshwater native to North America) were approximately 10 mg/L, with EC50 and NOECs ranging between 1.5 and 3 mg/L (Drottar and Krueger 2000; Ulhaq et al. 2013). Data from an acute test on the North American saltwater species sheepshead minnow (C. variegatus) for PFOS suggest this species is less sensitive than its freshwater counterparts, with EC50 greater than 15 mg/L (Palmer, Van Hoven, and Krueger 2002).
Chronic studies of PFOS toxicity are more limited; only two studies of P. promelas were identified and indicated a NOEC of approximately 0.3 mg/L and EC50 of 7 mg/L (Drottar and Krueger 2000; Oakes et al. 2005) for early life stage development.
Acute and/or chronic zebrafish toxicity studies are also available for PFOA, PFBS, PFNA, PFBA, and PFDA. Acute toxicity is highly variable, but in general, these compounds appear to be less toxic than PFOS, with LC/EC50 reported up to 3,000 mg/L; the exception to this generality is PFDA, where an EC50 of 5 mg/L was reported (Ulhaq et al. 2013). Chronic toxicity data in this species for these compounds are more limited, but the few available studies suggest chronic toxicity may occur at substantially lower concentrations; for example, Zhang et al. (2012) reported a LOEC of 0.01 mg/L for growth/weight was for PFNA.
16.3.2.4 Other Aquatic Chordates—Amphibians
Amphibians represent an alternative class of aquatic/semi-aquatic chordates for which PFAS toxicity studies have been conducted. There are currently limited PFAS toxicity data available for amphibians, but this class of organisms is becoming more widely studied. Nearly all the available amphibian studies entail acute studies in aquatic life stages (with mortality as the endpoint) on PFOS exposures to several species, including Xenopus laevis (African clawed frog), Rana pipiens (northern leopard frog), Rana nigromaculata (black-spotted frog), Pseudocris crucifer (spring peeper), Lithobates catesbeianus (American bullfrog toad), and Bufo gargarizans (Asiatic toad). Of these, R. pipiens L. catesbeianus, and P. crucifer are native to North America. Amphibian data are discussed in Section 7.2 for PFOS and PFOA. These studies indicate mortality is observed in amphibians at water concentrations over 10 mg/L, with chronic toxicity occurring at lower levels (although within the range of acute toxicities), and that PFOA is less toxic than PFOS.
16.3.2.5 Crustaceans
Freshwater
Acute
Acute toxicity data for freshwater crustaceans are focused on various daphnids (see USEPA 2023), with some data on freshwater shrimps such as the cherry shrimp (Neocaridina denticulate) (Li 2009). The largest number of publications are on the water flea, Daphnia magna, where 24–48-hour survival data are available for PFBA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, and PFOS. For most other freshwater species, acute toxicity data are limited to PFOA and PFOS.
Chronic
A smaller number of specific PFAS have been assessed for chronic toxicity in freshwater crustaceans. This includes PFOA, PFBS, PFOS, and GenX salt (ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate) (Drottar and Krueger 2001). The 21-day survival and reproduction assay with daphnids is reported for both PFOS and PFOA Section 7.2). A series of studies evaluated bioavailability of certain PFAS with respect to exposure cofactors such as dissolved proteins, organic carbon composition, and other solutes (Xia et al. 2015; Xia et al. 2015; Xia et al. 2013). Although these studies do not estimate ECs or LCs for specified PFAS, they demonstrate that abiotic factors require consideration for exposure assessment and comparison across aquatic toxicity studies.
Saltwater
Acute
Two short-term studies (96 hours) provide some data on the acute toxicity of PFOS in marine crustaceans. The two tested species, both mysid shrimp, are Siriella armata and Mysidopsis bahia, for which NOECs of 0.550—1.25 mg/L (Drottar and Krueger 2000; Mhadhbi et al. 2012) were derived; however, the values for S. armata were based on nominal concentrations (Mhadhbi et al. 2012). Additional studies are needed to evaluate other PFAS, as well as to discern differences in acute toxicity among benthic, epibenthic, and pelagic crustacean species.
Chronic
There are very few laboratory-based chronic toxicity studies available for marine crustaceans. One study was a 35-day growth, reproduction, and survival assay for PFOS using mysid shrimps (Mysidopsis bahia) (Drottar and Krueger 2000); (see Section 7.2). Simpson et al. (2021) more recently conducted a comprehensive study of PFOS sediment, overlying water, and/or porewater exposure to various benthic marine/estuarine species, reporting chronic effects data for an amphipod (Melita plumulosa), a copepod (Nitocra spinipes), and a crab (Macrophthalamus sp.).
While there are multiple studies regarding the occurrence of PFAS in wild-caught crustaceans, there is little information on biological effects. There is one recent report on wild-caught eastern school prawn (Metapenaeus macleayi) that found associations between metabolomic profiles and certain PFAS, but the exposure history for the animals was unknown (Taylor et al. 2019).
16.3.2.6 Mollusks
Freshwater
Acute
For freshwater mollusks, toxicity studies of PFOA and PFOS are limited to five species: bladder snail (Physa acuta), fatmucket clams (Lampsilis siliquoidea), black sandshell mussels (Ligumia recta), Unio ravoisieri, and zebra mussels (Dreissena polymorpha) (Li 2009; Hazelton et al. 2012; Fernández-Sanjuan et al. 2013; Amraoui, Khalloufi, and Touaylia 2018). The fatmucket and black sandshells are native to North America, whereas the remaining three are nonnative to the U.S. or are invasive species, as in the case of zebra mussels. Following 24- to 96-hour exposures, Hazelton et al. (2012) estimated lower EC50s for PFOS than for PFOA in both fatmucket clams and black sandshell mussels, where larvae were more sensitive than juveniles toward PFOS.
Chronic
Assessments of chronic effects in freshwater mollusks are limited to a single study with water concentrations that were much higher than those detected at most remediation sites. Following a 28-day exposure to PFOS, Hazelton et al. (2012) observed impaired growth effects in larval and juvenile fatmucket clams as determined by reductions in larval viability (LOEC of 0.0045 mg/L) and impaired metamorphosis (LOEC of 0.0695 mg/L), respectively. Fernández-Sanjuan et al. (2013) evaluated the physiological responses of nonnative and invasive zebra mussels toward a mixture of PFOA and PFOS (0.001—1 mg/L, 10 days), but did not report any mortality.
Saltwater
Acute
Acute toxicity studies for marine mollusks are currently limited to PFOA, PFNA, PFOS, and PFDA. A larger body of literature exists on occurrence and tissue concentrations in mussels and oysters, but almost none of these assessed whole or suborganism level effects. Two 96-hour acute toxicity tests reported NOECs for mortality in Unio complamatus (20 mg/L) and eastern oyster (Crassostrea virginica) (18 mg/L) (Drottar and Krueger 2000).
Chronic
Chronic studies for sublethal effects of PFAS on bivalve mollusks are limited to a few marine species, including green mussels (Perna viridis), Mediterranean mussels (Mytilus galloprovincialis) and California mussels (Mytilus californianus). In vitro and in vivo studies of California and Mediterranean mussels offer some information relative to effects on xenobiotic transport proteins and enzymatic activities, respectively (Stevenson et al. 2006; Balbi et al. 2017; Gülsever and Parlak 2018), but do not quantify ECs, NOECs, or LOECs for whole-organism exposures. A series of studies using green mussels reported on multiple endpoints following 7-day exposures to PFOS, PFOA, PFNA, and PFDA. These studies evaluated biochemical markers of altered xenobiotic metabolism (Liu, Gin, and Chang 2014), genotoxicity (Liu et al. 2014), oxidative stress (Liu, Chang, and Gin 2014), and immunotoxicity (Liu and Gin 2018) and reported NOECs ranging from 0.010-0.100 mg/L (PFOA and PFNA) down to 0.001-0.01 mg/L (PFOS and PFDA). Simpson et al. (2021) reported chronic no-effects levels for two species of bivalve mollusks (Tellina deltoidalis and Soletellina alba) of 0.22 mg/L and ≥ 0.85 mg/L, respectively.
16.3.2.7 Aquatic Insects
Freshwater
Toxicity data for aquatic insects (for example, midges, mayflies, dragonflies) are available in the literature, although they mainly stem from acute studies of PFOS, PFOA, and PFNA in the freshwater midge species Chironomous riparius and C. tentans (see Section 7.2.2.1). Of the aquatic invertebrate species, the chironomids are currently reported as having the highest sensitivity to PFOS (MacDonald et al. 2004). Acute effects are observed to generally occur at water concentrations of approximately 60 mg/L or greater, with chronic effects induced at concentrations less than 0.002 mg/L (see Section 7.2.2.1).
Toxicity data for other orders of aquatic insects are very limited, although some studies indicate that the odonates may also be highly sensitive to PFAS exposures. Van Gossum et al. (2009) found behavior changes following long-term (4-month) exposure of PFOS to damselflies (Enallagma cyathigerum) with NOECs reported from 0.010 mg/L to 0.100 mg/L. Bots et al. (2010) conducted a lifetime exposure study of PFOS on E. cyathigerum, finding adverse effects on egg development, larval hatching, development and survival, metamorphosis, and body mass, with NOECs ranging from less than 0.010 mg/L (for metamorphosis) to over 10 mg/L (for egg hatching success).
Saltwater
No toxicity information was found on marine insects.
16.3.2.8 Algae/Vascular Plant Data
Section 7.2.4.1 provides a summary of aquatic plant toxicity data. Data on the toxic effects of PFAS on aquatic plants are limited to studies that evaluated PFOS exposures on several algal/microalgal species, on duckweed (Lemna gibba), and on watermilfoil (Myriophyllum spicatum). Acute toxicity of PFOS in freshwater aquatic plants has been found to range from approximately 30–100 mg/L, with chronic values generally about tenfold lower.
In a chronic study on a saltwater species of diatom (Skeletonema costatum) Desjardins et al. (2001) reported observed effects at approximately 3 mg/L. Simpson et al. (2021) reported a chronic NOEC of > 4.2 mg/L for growth rate as an endpoint.
16.3.3 Aquatic-Dependent Wildlife
This section considers aquatic-dependent wildlife (primarily birds and mammals) in developing surface water criteria for PFAS. It is important to note that the development of such criteria is still in its infancy because (i) there are few laboratory or field studies with data on the toxicity of PFAS to wildlife, (ii) data of sufficient/appropriate quality on the concentration of PFAS in the diet of aquatic-dependent wildlife are limited, and (iii) the unique properties of PFAS make the modeling of food chain uptake complicated. The following sections briefly discuss why aquatic-dependent wildlife should be considered and present methods that can be used to derive surface water quality criteria for their protection.
16.3.3.1 Why Consider Aquatic-Dependent Wildlife?
As described in Section 16.3.1, the focus for deriving surface water quality criteria is primarily on protecting aquatic life (for example, plankton, benthic invertebrates, fish, shellfish). However, it has long been recognized that wildlife species may be more sensitive than aquatic species toward certain contaminants as a result of dietary exposure, particularly if the contaminant is bioaccumulative (USEPA 1985, 1989, 2005).
Because some PFAS are known to be bioaccumulative (see Section 5.5), it cannot be assumed that surface water quality criteria derived for the protection of aquatic life will also be protective of aquatic-dependent wildlife. Protection of aquatic-dependent wildlife is of importance at contaminated sites, particularly for wildlife with smaller home ranges that coincide with the extent of PFAS impacts (Conder et al. 2020; Divine et al. 2020). In developing risk-based screening levels (RBSLs) for different classes of receptors, Divine and coworkers found that for some PFAS, RBSLs for aquatic-dependent wildlife are lower than RBSLs for aquatic life. Lastly, due to the long-range transport for some PFAS (see Section 5.3), there could be a need for surface water quality criteria protective of aquatic-dependent wildlife in remote areas where management actions to address human exposure may not address ecological exposures.
16.3.3.2 Surface Water Quality for the Protection of Wildlife
The principal approach to developing protective surface water quality for wildlife is to use standard desktop wildlife exposure models to solve for a media concentration that results in exposure being equal to a selected toxicity threshold. In modeling exposure, such an approach either uses measured contaminant concentrations in the tissue of the prey/forage or relies on BAFs (to estimate prey/forage tissue concentrations). The same toxicity threshold can be used in either case. Another approach is to determine a critical concentration in the tissue of a prey item that is protective of the wildlife receptor. The advantage of this “body burden” approach is that it avoids the uncertainties associated with relying on BAFs; however, it entails the collection of site-specific tissue data for implementation.
These two approaches are discussed in the following sections.
16.3.3.3 Calculation of a Protective Surface Water Quality Value for Aquatic-Dependent Wildlife
In 1995, USEPA published the Great Lakes Water Quality Initiative (GLI) Technical Support Document for Wildlife Criteria (USEPA 1995). The GLI Technical Support Document provided technical information on the derivation of surface water quality criteria to protect birds and mammalian wildlife. The method is similar to that used to derive noncancer human health criteria and relies on the use of BAFs in a food chain model to back-calculate a surface water value (USEPA 2017). USEPA has used this method for chemicals such as DDT, PCBs, 2,3,7,8-TCDD, and mercury, for which aquatic life-based criteria were modified to become more stringent in order to incorporate adverse effects to wildlife (USEPA 1995). States and tribes bordering the Great Lakes, as well as other states, such as California, have since adopted this approach to derive aquatic-dependent wildlife surface water quality criteria.
The GLI Technical Support Document (USEPA 1995), Section III, provides the equations needed to calculate a protective surface water quality value for birds and mammals from exposure via food and water ingestion (see text box).
Standard Equation for Derivation of a Protective Surface Water Quality Value for Aquatic-Dependent Wildlife
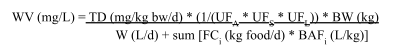
Where:
WV = wildlife value in milligrams of substance per liter (mg/L)
The bird WV is the geometric mean of the WVs calculated for the three birds and the mammalian WV is the geometric mean of the WVs calculated for the two mammalian wildlife species.
TD = test dose in milligrams of substance per kilogram per day (mg/kg-d) for the test species. This shall be either a NOAEL or a LOAEL (expressed either on a wet weight or dry weight basis for consistency with FC).
UFA = uncertainty factor (UF) for extrapolating toxicity data across species (unitless). A species-specific UF shall be selected and applied to each representative species, consistent with the equation.
UFS = UF for extrapolating from subchronic to chronic exposures (unitless)
UFL = UF for LOAEL to NOAEL extrapolations (unitless)
BW = body weight in kilograms (kg) for the representative species
W = daily volume of water consumed in liters per day (L/d) by the representative species
FCi = daily amount of food consumed from the ith trophic level in kilograms per day (kg/d) by the representative species (expressed either on a wet weight or dry weight basis for consistency with TD)
BAFi = bioaccumulation factor for the ith trophic level in liters per kilogram (L/kg)
By extension, this method can be used in the development of surface water values for PFAS. The GLI approach uses five representative species (bald eagle, herring gull, belted kingfisher, mink, and river otter), because these species are likely to be subjected to the highest exposure from bioaccumulative contaminants through the aquatic food web. However, depending on the conceptual model under evaluation, it might be more appropriate to select different representative species (for example, sandpiper species given their relatively high incidental ingestion of sediment). Giesy et al. (2010) provided good examples of using the GLI approach to calculate surface water criteria for trophic level IV predatory birds for two compounds, PFOS and PFBS.
The GLI Technical Support Document includes tables with values for the exposure parameters (body weight, water and food ingestion rates, as well as prey/forage trophic level). Other sources of receptor-specific exposure parameters can be found in USEPA’s Wildlife Exposures Factor Handbook USEPA (1993), Conder et al. (2020), and Divine et al. (2020). However, it is a best practice to select exposure parameters that are most representative of the populations living within the area to which the criteria will be applied. Climate, temperature, habitat, and many other factors specific to the region underlying the studies that are the basis of the exposure factors can vary significantly.
Key PFAS-specific components to this approach are the selection of, and sources for, BAFs to estimate prey or forage tissue concentrations, and the wildlife toxicity data for PFAS (no observed adverse effect levels [NOAELs] and lowest observed adverse effect levels [LOAELs]).
As noted in Section 16.2.1.4, given the influence of BAFs on calculation of a water quality value, their use and applicability need to be carefully considered. Information on a literature review and selection of BAFs is provided in Section 5, with a compilation of BAFs and their sources presented in Table 5-1 (see link in Section 5.5).
As noted, there are currently few PFAS toxicity data for wildlife in the scientific literature or standard toxicity databases. Furthermore, given the minimum toxicity database requirements for developing Tier I criteria (see USEPA (1995)), it is unlikely these requirements will be met for individual PFAS at the current time. Note that the GLI technical support document does provide guidance on developing Tier II wildlife values for contaminants with limited toxicity data (USEPA 1995). Conder et al. (2020) and Divine et al. (2020) are good sources of PFAS toxicity reference values (TRVs) for avian and mammalian wildlife that not only include tables of recommended values, but also provide guidance on reviewing toxicity studies reported in the literature and selecting the values.
16.3.3.4 Calculation of a Protective Prey/Forage Tissue-Based Value
USEPA published a science advisory board consultation document on tissue-based criteria for “bioaccumulative” chemicals as proposed revisions to aquatic life guidelines (USEPA 2005). The approach is based on the recognition that for bioaccumulative chemicals, there is a need to develop criteria that account for multiple routes of exposure such as the diet, sediment, and water.
The approach is similar to that for developing a water-based value in that it is based on a daily dietary dose of a chemical that is protective of most sensitive species and integrates it with exposure potential to estimate a chemical concentration in the dietary tissue of representative species, also referred to as a wildlife value (WV). The advantage of this approach over the water-based approach is that it eliminates the uncertainty associated with using BAFs but requires the sampling of appropriate prey biota for implementation.
The equation in the text box below used to develop a tissue-based WV is based on the GLI model for aquatic-dependent birds and mammals but expressed as the chemical concentration in the diet rather than in water (see USEPA (2005), Section 4.4). As discussed above, sources of exposure information can be found in USEPA (1993, 1995), Conder et al. (2020), and Divine et al. (2020).
Note that if the target aquatic-dependent wildlife is exposed via several trophic levels, a WV will need to be calculated for each trophic level using BAFs, or if available, applicable BMFs for the targeted aquatic-dependent wildlife receptor (see Section 5.6). This will then guide which species of prey/forage to target when monitoring for compliance.
Standard Equation for Derivation of a Protective Prey Tissue Value for Aquatic-Dependent Wildlife
![]()
Where:
WVww = wildlife value expressed as the chemical concentration in the diet of each representative species measured as wet weight
TD = test dose expressed as daily dietary dose (wet weight) from selected study, either a NOAEL or a LOAEL
UF = uncertainty factors for interspecies variation (UFA), subchronic to chronic (UFS), and LOAEL to NOAEL (UFL)
BW = body weight of a representative species
FCi = amount of daily food consumed for each species from the ith trophic level
Because this approach was developed to address the bioaccumulation of lipophilic chemicals, it assumes the chemical contribution from water is negligible, which, as discussed in Section 5.5.2, may not be the case for some PFAS. Furthermore, it does not consider dietary intake via the incidental ingestion of sediment, which as noted above, can be included depending on the species of wildlife being evaluated. As indicated by Larson, Conder, and Arblaster (2018), incidental sediment ingestion could be a significant contributor to exposure to aquatic-dependent wildlife.
16.4 Sampling and Analysis
This section reviews the collection and analysis of surface water, biota, and surface sediment from aquatic systems. In most instances, the precautions taken to minimize biasing the concentrations of PFAS in the samples from sampling equipment and/or sampler protective clothing, among others, are the same as those described in Section 11.1 when sampling groundwater, surface soils, or fish. There are some unique aspects about where to collect a surface water sample that are discussed in this section. For analysis, the methods used for PFAS in drinking water, soil samples, plant material, and groundwater described in Section 11.2 are also used for surface water, biota, and surface sediment. Issues and recommendations unique to sampling PFAS-containing foam are covered in Section 16.5.
16.4.1 Surface Water Sampling
Surface water bodies include, but are not limited to, oceans, bays, estuaries, lakes, streams, ponds, creeks, springs, wetlands, reservoirs, and artificial impoundments. The sample collection for PFAS from a surface water body is essentially the same as for other chemicals or pollutants. Standard operating procedures for sampling equipment have the same limitations, such as PFAS-free samplers, that are used for collection of samples from potable water systems or groundwater monitoring wells. See Section 11.1 for general sampling techniques and equipment requirements for PFAS sampling, including examples of PFAS-specific sampling protocols, and Section 11.2 for analytical methods and techniques.
In addition to USEPA’s Compendium of Superfund Field Operations Methods (USEPA 1987) for general guidance on sampling surface water, Michigan (MI EGLE 2021; MI DEQ 2018) and New York (NY DEC 2021) have recently developed guidance specifically for PFAS. Some examples of sampling consideration in a moving water body such as a creek include:
- sampling far enough downstream from the suspected source to allow for adequate mixing
- collecting samples from the upstream side of the sampler to minimize compromising the sample
- collecting the sample from mid-depth in the thalweg (that is, along the natural direction of water flow, below the air/surface water interface but above the surface water/surface sediment interface)
- depending on your sampling objectives, collecting samples from multiple depths to provide the necessary information. This is due to the potential for stratification of PFAS concentrations through the water column and the location of the receptors of concern (benthic organisms or fish).
Due to chemical properties of PFAS, concentrations in the surface water near the discharge location may be higher at or near the surface. Collecting samples only from the surface may inform you of the worst-case scenario but may not provide information on locations away from the discharge point, such as at a water intake for domestic and industrial use. The same concern applies to PFAS-containing foam. Including this foam in the sample will bias the PFAS concentration high due to the elevated concentrations in the foam (see Section 16.5.5).
Additional considerations for surface water sampling during site characterization are noted below in Section 16.4.5.
16.4.2 Biota Sampling
Because some PFAS are known to accumulate in aquatic biota and in some instances may drive the development of surface water quality criteria (see Sections 16.2.2.2 and 16.3.3), sampling for biota may be an important component of a monitoring program or health assessment. The species of biota to collect and the tissue types to sample will depend on the study objectives. For example, a human health–based study should focus on collecting species within a size range targeted by recreational anglers or crabbers (NJDEP 2018), while an ecological-based study should focus on species representing different trophic levels, as well as benthic and pelagic habitats, and be of a size range targeted by piscivorous birds and mammals. In addition, because it has been shown that some PFAS bioaccumulate to a higher degree in the blood and liver compared to the flesh (see Section 11.1.7.8), collecting and analyzing different tissue types might be an important consideration in a study design.
General guidance on the use of different sampling techniques for fish and shellfish such as gill nets, seines, trawls, and electrofishing, can be found in USEPA (2000), and many states have their own guidance, which should be considered. When sampling biota for PFAS analysis, the additional precautions described in Section 11.1 should also be followed, and some states, such as Michigan, have developed their own PFAS-specific guidance (MI DEQ 2019). For example, collected biota should be wrapped in HDPE or polypropylene bags and/or aluminum foil, and stainless-steel tables, knives, and weighing scale hooks should be used for sample processing (in the field or in the lab), as well as untreated wooden cutting boards. Given that PFAS are widely present in the environment and human-made materials, it will be important to collect equipment blanks during sample collection and processing. Close coordination with the analytical laboratories will be needed to ensure similar PFAS-specific precautions are followed at all times (see Section 11.2).
Additional considerations include sampling surface water and surface sediment within the same area from which the biota are collected. While it is recognized that many aquatic biota are migratory or exhibit extended home ranges, this information will help support identification of PFAS sources as well as potentially the development of site-specific BCFs, BAFs, and biota-sediment concentration factors (BSAFs). BSAFs represent the amount of PFAS taken up by an organism from the sediment and are measured as the ratio of the concentration in an organism to the concentration in sediment. BSAFs are typically developed for those organisms in close contact with surface sediment, such as benthic and epibenthic invertebrates, as well as benthic fish (Figure 17-1).
16.4.3 Sediment Sampling
Because sediment can be a contaminant sink, a transport mechanism, and a source of contaminants to a surface water body and to benthic organisms, it may be necessary to sample sediment for PFAS to support an understanding of its contribution to the surface water quality and/or biota tissue concentrations. Conventional sediment sampling and coring techniques and protocols can generally be used to obtain samples for analysis of PFAS. Section 11.1 describes sampling protocols for all types of samples, with additional detail for porewater in Section 11.1.7.4 and sediment samples in Section 11.1.7.7
Examples of seven different sampling protocols typically used are detailed below:
- Where the sediment is accessible and can hold its form without collapsing, a corer or “Dormer Piston” sediment sampler could be used.
- When collecting samples from shore or wading, the sample should be collected from the upstream side of the sampler to minimize potentially compromising the sample from stirred-up sediment or from a waterproof coating on waders.
- Sediment core samples are collected directly from single-use liners and are not reused.
- There can be sites where the sediment is accessible but either the sediment is sloppy and would not hold its form, or there is a high density of tree roots or boulders and a corer or piston sampler would not be feasible. In these situations, a stainless-steel trowel could be used to collect surface sediment samples.
- For subtidal sediments, the depth below the sediment surface from which the sample is required determines what equipment is needed. For surface sediment samples, devices such as a modified van Veen grab, Ekman grab, or Ponar grab sampler can be used, while for deeper subsurface samples, devices such as a vibracorer should be used. This nondedicated equipment (equipment used for more than one water body or location) should be verified as PFAS free, and the sampling program should include collection of equipment blanks.
- For sampling subtidal sediments, the depth from which the sample is required dictates what specialized sampling equipment is needed.
- Samples should be collected in HDPE wide-mouth bottles provided by the laboratory, and fitted with an unlined (no Teflon) polypropylene screw cap. A minimum of 50 g of sample is needed. Field observations, including sediment type, texture, and color, should be recorded.
16.4.4 Analytical Methods
Analytical methods for the analysis of PFAS in a surface water share many of the same components as those used for PFAS in other non-drinking water media. A discussion of these analytical methods is found in Section 11.2. Most surface water samples for PFAS are analyzed by Modified USEPA 537.1 and use the DOD’s Quality Systems Manual (QSM) for Environmental Laboratories, Version 5.3, Appendix B, table B-15 (USDOD 2019), providing the most current and comprehensive set of quality standards for PFAS analysis. In June 2019, USEPA validated USEPA SW-846 Method 8327 for the analysis of PFAS in surface water, groundwater, and wastewater (USEPA 2021). Method 8327 is available for use but has not yet been fully incorporated into SW-846. Method 8327 has not been widely used because it creates laboratory cleanup and accuracy issues and is not accepted by DOD.
16.4.5 Site Characterization
Conducting site characterization of PFAS at a site with a surface water body should begin with determining the beneficial uses as described in Section 16.1.1. Those beneficial uses should be used to select the types of samples to be collected. For example, if evaluating PFAS-containing foam, sample collection will be required near the surface; if evaluating potential impacts to benthic organisms, samples closer to the sediment/water interface should be collected, and if evaluating pelagic fish, it may be necessary to collect a surface water sample integrated throughout the water column. Point sources such as stormwater discharge pipes or a publicly owned treatment works (POTW)discharge should be located and potentially targeted for sampling to evaluate sources of PFAS to the water body. Establishing the locations of natural and human-made water inflow and outflow points to the water body will also help guide sample points and potential exposure sites. Groundwater/surface water interaction may also play a role in establishing the conceptual site model and locating places to sample. Section 10 contains more specifics regarding site characterization and development of a conceptual site model.
16.5 Surface Water Foam
PFAS-containing foam (discussed in this section) is differentiated from AFFF (discussed in Section 3) in that it is the result of dissolved-phase PFAS in surface waters that have been agitated by wind or wave action and aggregated into a mass at or above the surface of the water, irrespective of the PFAS source type. As noted in Section 16.2.2.4, the presence of foam does not necessarily indicate the presence of PFAS. Foam can form naturally when the surface tension of water decreases and the concentration of organic matter, such as dissolved organic carbon (DOC), increases and is mixed into the water. The decomposition of organic material into water, or a storm event mobilizing existing organic material, can lead to the natural formation of foam on surface water. For example, a study of PFAS and DOC in foam forming on a freshwater lake in Michigan showed that PFAS made up less than 0.1% of the DOC present in the foam samples, which indicated that DOC was the primary cause of the foam and not the presence of PFAS (Schwichtenberg et al. 2020).
This section discusses the formation of PFAS-containing foam, the characteristics of, and stratification within the foam, a brief discussion of analytical methods for foam, and the enrichment of PFAS concentrations in foam compared to the underlying water column. A case study on PFAS containing foam (the Minnesota Project 1007 Feasibility Study) is presented in Section 15.5.1.
16.5.1 Foam Above Water Interface
PFAS-containing foam may occur at and above the air-water interface on surface waters. In this context, surface water is subdivided and defined below. This subdivision describes vertically downward the air-water interface, surface micro layer (SML), neuston, and underlying water column. See Figure 16-2.
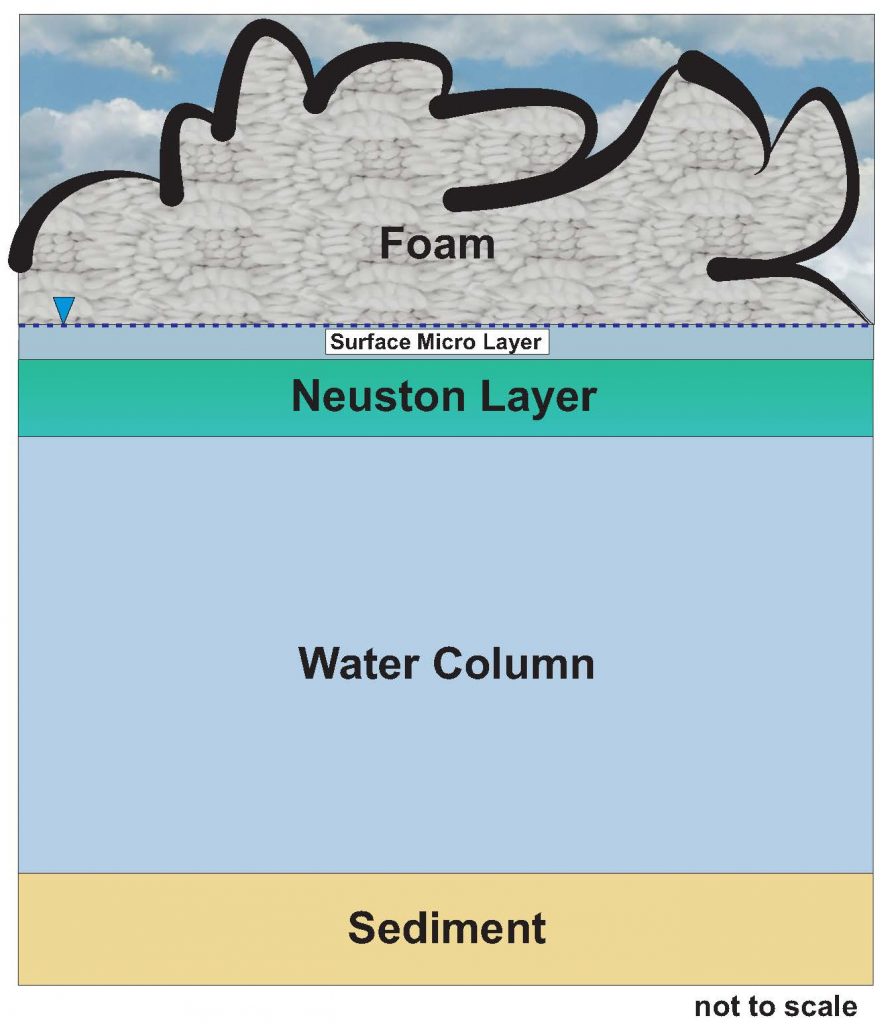
Although it is typical to find PFAS-containing foam near release source areas, it may also occur some distance away when surface water bodies are interconnected. Source proximity may impact the concentration of PFAS analytes in the foam column itself. However, concentrated PFAS-containing foam may occur on surface water bodies containing low to nondetect PFAS concentrations immediately below the foam itself some distance from the source areas (MPCA 2020; Schwichtenberg et al. 2020). Underlying partitioning considerations are discussed in Section 5.2.2, Considerations for PFAS Partitioning.
Variable surface water depth, flow conditions, and co-contaminant mixtures may affect the presence, aggregation, and physical movement of PFAS-containing foam in the SML and above the air-water interface. These factors may be considered when evaluating fate and transport of PFAS-containing foam, as it may partition back into solution in the water column as it moves with the flowing water. A foam column at and above the surface may be affected by physical forces, including precipitation, wind, and wave action, causing it to separate and travel as smaller foam source material “islands” or collapse and dissolve back into the water column as it travels with the wind and waves.
16.5.2 Foam Characteristics
PFAS-containing foam has wide-ranging visual and textural appearances. These characteristics range from deflated, dry, or aged in appearance, as small aggregations of bubbles accumulating into larger islands of billowed piles, frozen in standard bubble foam appearance or frozen in thin sheetlike membrane appearance, frozen on top of snow and ice, dark coloration due to detritus or organic content, and bright white, with an aerated shaving cream or whipped cream texture.
“High turbulence generated foam tends to be fluffy, actively regenerates, and does not appear to contain high levels of organic material; while older, deflated foam does not actively regenerate and appears to be rich in organic or non-organic particulates. Foam observed along creeks and streams tend to vary in appearance and can occur with any combination of the above-listed characteristics. Wind-generated foam has only been observed on lakes, has generally been white and fluffy, and has actively accumulated along a downwind shoreline. Both organic-rich foam and unfrozen foam with a wrinkled appearance have been observed to accumulate along ice dams or ice shelves” (MPCA 2020).
16.5.3 Stratification Within Foam
Surface water foam is known to be laden with bacteria, detritus, and other naturally occurring material. However, stratification of concentrations of PFAS within the foam column or pile may be due to a number of physical and chemical conditions. For example, co-contaminants may impact the degree of stratification in the surface water column and the foam itself. Additionally, the foam may be aged and in a dry condition toward the top of the foam column/pile, creating a concentration gradient toward the base of the column/pile.
Sampling should be conducted so as to maximize consistency and reproducibility during PFAS surface water sampling to understand PFAS stratification in the surface water column and the foam. The Michigan Department of Environment, Great Lakes, and Energy (EGLE) has published a surface water foam sampling guidance, a surface water foam study report, and has published other PFAS sampling guidance (MI EGLE 2019, 2021, 2021).
As of the date of this publication, the state of Minnesota is working with technical support from the USEPA Office of Research and Development to evaluate foam sampling techniques aimed at understanding the concentration variability within the foam, at the surface micro layer, and the underlying near-surface water column.
16.5.4 Frozen Foam
PFAS-containing foam has been confirmed in frozen form on surface water in Minnesota, Michigan, and Wisconsin (MPCA 2019). Appearance of the foam is difficult to distinguish from snow and ice along stream or lake embankments. PFAS-containing ice has been confirmed in Michigan and can visually take the form of thin film-type islands or within massive ice (MPCA 2020). The ice appears to stratify vertically in concentration where located in massive ice. Confirmation of the degree of stratification in massive ice is an on-going study at this time. Early results from Michigan indicate it appears to increase in concentration with elevation in the ice cores, indicating the ice/air interface may be where the highest concentrations exist, similar to the propensity for PFAS to travel at the air/water interface.
16.5.5 Surface Micro Layer
As previously discussed, PFAS in solution tends to accumulate at the air/water interface, also known as the surface water micro layer or SML. The SML in this context, is generally understood to mean the layer in contact with ambient air and is approximately 50µm thick (MPCA 2020). Variability in sample collection techniques and the extent to which this interface is included or omitted in the collection of a bulk water sample may result in biased analytical results. This can further lead to incorrect conclusions about PFAS concentrations in surface waters at source areas and in the downgradient direction. Where the SML is not characterized and only the deeper water column is sampled, PFAS concentrations may be biased low . The SML may be the location of the highest PFAS concentration in the water column. Omitting collection of the SML could potentially lead to an inaccurate assessment of the risk to human and ecological receptors. Therefore, when preparing a sampling plan, it is important to consider the depth of the water column that is most relevant to the human and ecological receptors that may be exposed to PFAS; different receptors may require unique sampling strategies. At this time, methods to characterize PFAS concentrations and the nature of exposed biota in the SML are still under study (see SERDP project ER19-1205, Field et al. 2021).
Due to the physical and chemical properties of PFAAs, including their hydrophilic heads and hydrophobic tails, a given molecule may preferentially exist within the SML. Section 4 includes information about PFAS physical and chemical properties. Sampling the SML in relative isolation from the neuston layer below it or the foam column above it may become an important factor in accurate representation of the concentrations emanating from the SML into foam above when physical agitation occurs. The Michigan EGLE guidance for sampling PFAS-containing foam provides a starting point to understanding the considerations of this sampling protocol in varied circumstances (MI EGLE 2019). Michigan EGLE has also published other PFAS sampling guidance that may be relevant for a project (MI EGLE 2021).
16.5.6 Neuston Layer
The neuston layer may be defined, for the purposes of studying PFAS-containing foam, as the zone directly underlying the SML that is typically enriched with biological life and aligns with the larger definition by Wurl et al. (2017), which was related to strata thicknesses. Wurl et al. (2017) indicated:
“The sea surface microlayer is the boundary interface between the atmosphere and ocean, covering about 70% of the Earth’s surface. The SML has physicochemical and biological properties that are measurably distinct from underlying waters. Because of its unique position at the air-sea interface, the SML is central to a range of global biogeochemical and climate-related processes. Historically, the SML has been summarized as being a microhabitat comprised of several layers distinguished by their ecological, chemical and physical properties with an operational total thickness of between 1 and 1000 µm.”
“While this 1,000 µm SML definition is large enough to encompass the neuston, the refined definition of 50 µm thick SML provided above is considered the state-of-the practice for the purposes of understanding and evaluating PFAS-containing foam and layers of significance immediately underlying it. Liss and Duce (1997) clarify the neuston below the 50 µm SML as two distinct biological layers, “[The] neuston can be divided into epineuston and hyponeuston. The epineuston includes more than 40 species of water striders, Halobates, inhabiting the open ocean and coastal areas. The hyponeuston are organisms in the surface centilayer including hydrozoa, mollusks, copepods, isopods, decapod crustaceans, fishes, and the seaweed Sargassum.”
Section 11.1.7.3 includes generalized surface water sampling guidance references. For PFAS-containing foam on surface water, it appears that the neuston layer is of particular importance in understanding dissolved-phase PFAS within the water column that may be available to the SML for aggregation and concentration into foam at the air-water interface. The neuston may also provide insight into the stratification of PFAS in the water column below the interface.
16.5.7 Analytical Methods
Analytical methods for the analysis of PFAS-containing foam are essentially the same as those used for PFAS in other non-drinking water media. A discussion of these analytical methods is found in Section 11.2. As with all non-drinking water samples, there is no USEPA-certified method for the analysis of PFAS at the time of this publication. Efforts are being undertaken to develop the various analytical methods for the various non-drinking water media. Since there may be very high levels of PFAS in PFAS-containing foam, the laboratory should be warned of this potential so that it can take the necessary precautions during analysis. In the Minnesota case study (MPCA 2020), a commercial laboratory analyzed PFAS-containing foams using their proprietary method of LC/MS/MS. These foam samples were collected following the Michigan foam sampling guidance noted above, with caution taken to prevent dilution of the sample by minimizing contact with the water during foam sample collection.
16.5.8 Enrichment Factors
Enrichment factors are defined for the context of this document as “the calculated fold increase (or decrease) of PFAS concentrations in foam compared to the co-located surface water sample. These values are unitless and are determined by dividing the foam concentration of a specific PFAS compound by the concentration of the same compound detected in the surface water sample. The foam enrichment factor for a given PFAS compound indicates whether that compound is preferentially concentrating into the foam from the bulk water column” (MPCA 2020).
As reported by McMurdo et al. (2008), “Kaiser et al. (2006) observed that surface foam created by bubbling air through an aqueous solution was enriched in perfluorooctanoate PFO by up to 3.2 times.” However, simple enrichment factors have been documented in PFAS analytical samples from sites in Minnesota with factors ranging up to >32,000 times (MPCA 2020).
Currently, the extent to which the presence of foam may deplete PFAS concentrations in surface water is unclear. Enrichment factors of short-chain versus long-chain PFAS differed in one Minnesota case study (MPCA 2020) where 33 PFAS analytes were provided. With few exceptions, “short chain PFAS did not tend to enrich into the foam. Often the four, five, and six-carbon length chains were not detected in the foam samples. If they were detected, their concentrations were lower compared to concentrations measured in the surface water sample. Long-chain PFAS were found to have relatively higher foam-to-water enrichment factors than short chain PFAS. In a majority of the samples, 2-(N-ethyl-perfluorooctane sulfonamide) acetic acid (ETFOSAA) and perfluorooctanesulfonamide (PFOSA) had the largest foam enrichment” (MPCA 2020).
The ability of surfactants to concentrate in surface water foam is well known, but may be particularly of interest near source areas due to the ability of PFOA to partition into air circumstantially. Both terminal PFOA and PFOS may become entrained in these foams at high concentration, posing particular risk to ecological receptors in the neuston, SML, and those species reliant on the biological life above and below these zones. In support of this notion, Battelle (2018) indicated, “Foams in the natural environment are metastable and generally dissipate within seconds to days. Compositionally, foams are very similar to the SML, they are formed from the air entrapment on SML and they destabilize back to form SML. Compared to bulk water, foams are significantly enriched in many dissolved and particulate components, including particulate and organic matter, clay minerals, lipids, hydrocarbons, proteins, bacteria, hydrophobic contaminants, and heavy metals. As such, foams can provide a mechanism for fast transport of the contaminants through aquatic systems and a potential exposure pathway for aquatic animals and humans.” Due to concerns over potential adverse health effects from exposure to PFAS containing foam, the Michigan Department of Health and Human Services (MDHHS) recently issued a recommendation advising residents and visitors to avoid foam on Michigan waterbodies (MPART 2023).
16.6 Effluent Limits for PFAS
This section provides information about the current status of effluent limits for PFAS from discharges of wastewater to surface water.
16.6.1 Introduction
The protection of surface water quality from the impacts of discharges from publicly owned treatment works (POTWs) and industrial wastewater treatment works is based on the establishment of effluent limits for pollutants in the discharges from those facilities. The effluent limits are enforced through National Pollution Discharge Elimination System (NPDES) permits. Those effluent limits are developed by establishing technology-based (TBELs) and water quality-based (WQBELs) effluent limits for a specific pollutant and using the most restrictive value of the two for the final effluent limit in the permit.
Effluent limits are also informed by effluent limit guidelines (ELGs) that are national wastewater discharge standards developed by USEPA on an industry-by-industry basis. These are technology-based regulations that are intended to represent the greatest pollution reductions that are economically achievable for an industry. The standards for direct dischargers are incorporated into NPDES permits issued by states and USEPA regional offices and permits or other control mechanisms for indirect dischargers (https://www.epa.gov/eg/learn-about-effluent-guidelines).
As of the date of this document, there are no USEPA-established ELGs for PFAS. USEPA outlined an approach for establishing ELGs for select PFAS in its PFAS Strategic Roadmap (USEPA 2021) and the agency released its Effluent Guidelines Program Plan 15 in 2023 (USEPA 2023 (see Section 16.6.5). Currently, only North Carolina has an NPDES permit with TBELs for PFAS. These TBELs were established using Best Professional Judgment, (USEPA 2010), the process used when ELGs are not available. Minnesota has adopted an NPDES permit with WQBELs for PFOS (see Section 16.6.4.1).
16.6.2 ELG Development and Implementation
This section summarizes the state of practice for how technology-based ELGs are developed and used in the process for selecting NPDES permit effluent limits. This is followed by a discussion of the current state of knowledge regarding data collection and ELG development plans by USEPA for PFAS.
16.6.2.1 ELG Development
ELGs “represent the greatest pollutant reductions that are economically achievable for an industry” (USEPA 2021[2432]). ELGs for direct discharges to surface water are enforced through effluent limits established in NPDES permits issued by state and USEPA regional offices. For discharges to a POTW, the ELGs are enforced through a pretreatment program established by the POTW and enforced through its NPDES permit and the permits issued by the POTW for discharges into its collection system. For the process of using ELGs to develop TBELs for an NPDES permit, see section 5.2.1 of the Permit Writer’s Manual developed by USEPA (2010).
ELGs are developed from empirical data that represent the performance of various technologies and best management practices available to any given industry type (See Figure 16-3 for key steps in the process). For toxic and nonconventional pollutants such as PFAS, ELGs are developed based on Best Available Technology Economically Achievable (BAT) (USEPA 2010). The ELG based on BAT is established using the performance associated with the best control and treatment measures that facilities in an industrial category can achieve while also taking into consideration economic achievability as it relates to pollutant reduction benefits. Many other factors are also considered in establishing the ELG and not just for those based on BAT. These factors can either be numeric or non-numeric/narrative limitations based on use of a specific best management practice (BMP). For more information, see section 5.2.1.1 of the Permit Writer’s Manual (USEPA 2010).
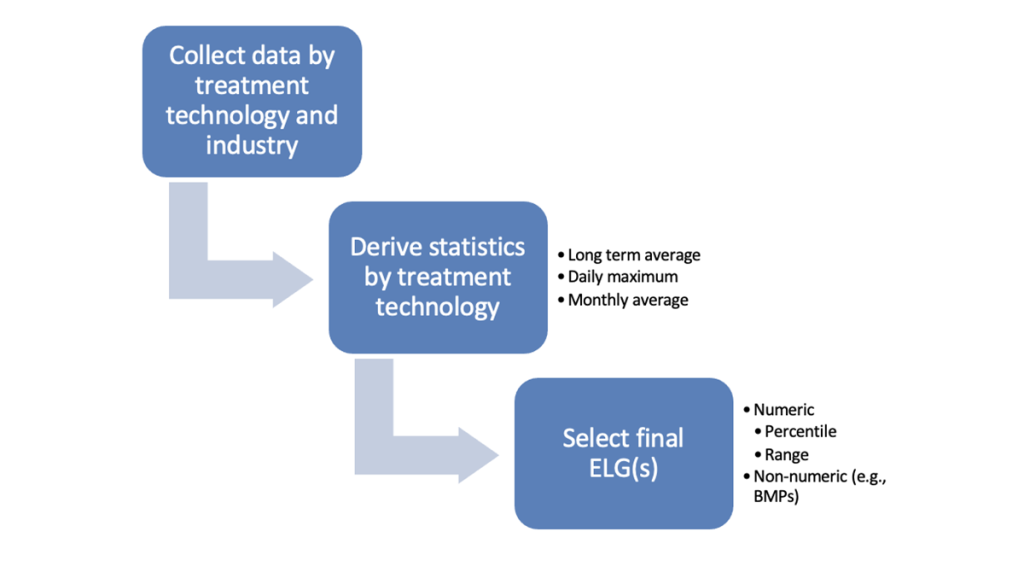
Figure 16-3. Key steps in the process of ELG development.
16.6.3 ELG Implementation
During the development of an NPDES permit, if it is found that the ELGs and/or state-established TBELs will not be sufficiently protective of water quality in the receiving water, then the CWA requires the development of WQBELs. WQBELs are developed so that the discharge authorized by the permit will not cause an exceedance of water quality standards that protect designated beneficial uses of the receiving water. See Section 16.1 for a discussion of beneficial uses for receiving waters. Some of the common beneficial uses include as drinking water, aquatic habitat, contact and non-contact recreation, and for commercial and sport fishing. Besides beneficial uses, the WQBELs take into account information about the receiving water that includes attributes such as ambient water quality, flow, dilution and additional discharges. For a comprehensive look at the WQBEL process, see Chapter 6 of the Permit Writer’s Manual (USEPA 2010).
In summary, the implementation of ELGs depends on whether the permit is for “direct discharge” (a direct discharge from the facility to ambient surface water) or “indirect discharge” to a POTW and permitted via a pretreatment program (Figure 16-4). For direct discharges, the ELG is typically used to set the TBEL, which is then compared against the ambient water quality standard (WQS) to see if it can be achieved by the TBEL alone. If implementing a TBEL would not lead to an exceedance of the WQS, then the TBEL could be used to set the final effluent limitation, after consideration of site-specific factors. If the TBEL might lead to a WQS exceedance, then development of a WQBEL is typically required for setting the final effluent limitation. WQBELs must consider not only federal requirements but any state-specific requirements. Note that for PFAS, only a few states have WQS (see the Water and Soil Regulatory and Guidance Values Table), so the availability of standards for use in setting WQBELs is as yet limited. As noted in Section 16.1, USEPA has published draft national recommended aquatic life criteria for PFOA and PFOS in freshwater (USEPA 2022; USEPA 2022). USEPA also plans to develop ambient water quality criteria for the protection of human health (based on drinking water and fish consumption) by Fall 2024. For more information on the potential risks to human health and ecological receptors from exposure to PFAS, see Section 9.1 and 9.2, respectively. For indirect discharges, pretreatment standards are selected from ELGs for existing sources, and for new sources.
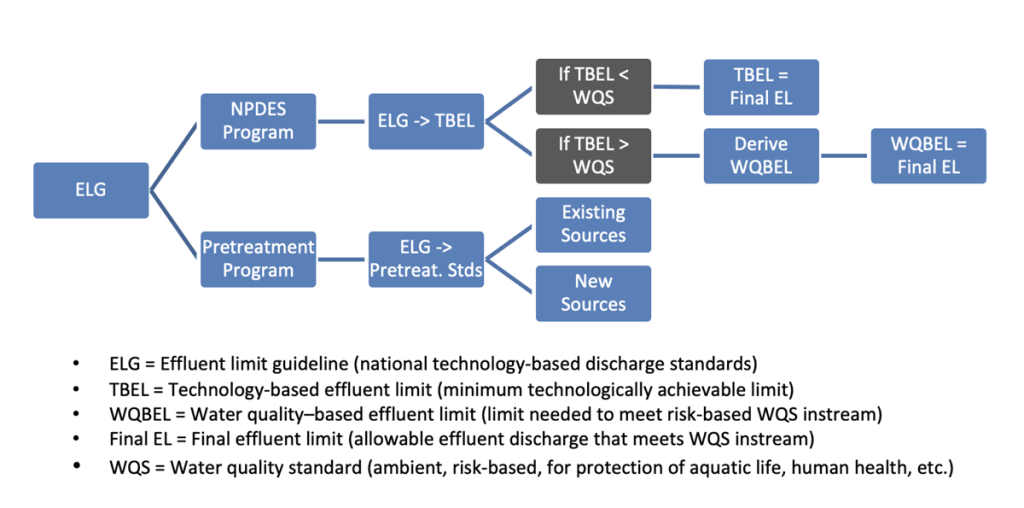
Figure 16-4. Schematic for implementation of ELGs
16.6.4 Status of ELGs and Effluent Limitations for PFAS
16.6.4.1 Existing ELGs and Effluent Limitations for PFAS
At the date of publication of this document, USEPA has not been established ELGs for PFAS. Therefore, while USEPA is in the process of developing ELGs and water quality criteria for PFAS, the agency released a memorandum in 2022 recommending that states develop permit-specific effluent limitations for PFAS based on TBELs (USEPA 2022[2650]). This was the approach taken by North Carolina in issuing an NPDES permit for the discharge of treated water from a groundwater remediation project (NC DWR 2022[2431] (see text box below). The PFAS limits developed for this NPDES permit are considered TBELs, unique for this discharge, and should not be assumed protective for a different NPDES permit.
North Carolina NPDES Permit for a Groundwater Remediation Discharge
Problem: PFAS in groundwater discharging to river—60% of PFAS load to river.
Solution: Capture groundwater seeps to river and treat to remove PFAS.
Primary indicator PFAS – HFPO-DA (GenX); PFMOAA (perfluoro-2-methoxyacetic acid); PMPA (perfluoromethoxypropyl carboxylic acid). Sufficient reduction of these shown to be indicative of sufficient reduction of total PFAS in discharge.
Discharger provided evaluation showing that granular activated carbon (GAC) could remove the 68 PFAS listed in the permit by at least 99%. Established as the TBEL.
Effluent limitation set at 99% reduction on a monthly mass basis using the three indicator PFAS to show compliance. Based on current concentrations—the expected effluent would be less than 122 ng/L HFPO-DA, 643 ng/L PFMOAA, and 132 ng/L PMPA.
When USEPA adopts PFAS surface water quality criteria, a reasonable potential analysis will be conducted and the permit will be reopened to include new effluent limits based on water quality if they are more stringent than the TBEL.
Minnesota has issued an NPDES permit for a municipal wastewater treatment facility with effluent limitations for PFOS based on Minnesota’s water quality standard for PFOS (MPCA 2022). The treatment facility discharges to a river. The effluent limitations for the permit, which are a daily maximum of 497 ng/L and a quarterly average of 287 ng/L, were developed based on a reasonable potential analysis. That analysis indicated a reasonable potential for exceedance of Minnesota’s water quality standard of 12 ng/L PFOS for a river. As the facility is undergoing an expansion, the limits are enforceable upon completion and startup of the expansion, but no later than 6 months prior to permit expiration. The 2013 permit also contains a limit on the mass loading of PFOS in biosolids applied by land application of 0.384 lbs/acre. This limit is not found in subsequent permit updates in 2018 and 2022. These values were developed on a site-specific basis and are not necessarily protective for a different discharge.
16.6.4.2 USEPA PFAS Data Collection for ELG Development
USEPA’s collection of data to support development of ELGs for PFAS has been primarily through its effluent guideline program plans (specifically Plans 14 and 15), a 2021 Advance Notice of Proposed Rulemaking (ANPRM), and a 2021 Multi-Industry PFAS Study. In addition, USEPA is collecting data from landfills to support possible development of PFAS effluent limits for landfill leachate, and from industrial discharges to POTWs for possible development of PFAS pre-treatment effluent limits. These activities are discussed below.
USEPA Effluent Guidelines Program Plan 14
In 2019, USEPA issued its Preliminary Effluent Guidelines Program Plan 14 (Preliminary Plan 14) (USEPA 2019) and a supporting report, The USEPA’s Review of Per- and Polyfluoralkyl Substances (PFAS) in Industrial Wastewater Discharge (USEPA 2019). The preliminary plan and the report discussed an initial review of PFAS discharges to surface waters and POTWs and concluded that “…little is known about the identity, frequency, or amount of PFAS compounds discharged in industrial wastewater.” The report recommended a follow-up study to collect data on PFAS manufacture, use, control, and discharge to surface water by industries USEPA determined to be likely dischargers of PFAS. These consisted of airports, organic chemical manufacturers, paper and paperboard manufacturers, and textile and carpet manufacturers. This became the Multi-Industry PFAS Study (see below). With publication of the final Plan 14 in 2021 (USEPA 2021), metal finishers were added to the Multi-Industry PFAS Study.
Advance Notice of Proposed Rulemaking
In March 2021, USEPA issued an Advance Notice of Proposed Rulemaking (ANPRM) titled “Clean Water Act Effluent Limitations Guidelines and Standards for the Organic Chemicals, Plastics and Synthetic Fibers Point Source Category” (USEPA 2021). The ANPRM noted that USEPA was “initiating further data collection and analysis to support potential future rulemaking under the CWA relating to ELGs, pretreatment standards, and new source performance standards applicable to the OCPSF point source category.”
Multi-Industry PFAS Study
As an outcome of USEPA’s effluent guideline program, The Multi-Industry PFAS Study (USEPA 2021) focused on collecting, compiling, and reviewing information and data on PFAS in discharges from industries in the following point source categories:
- Organic chemicals, plastics and synthetic fibers (OCPSF) manufacturers and formulators
- Metal finishing
- Pulp, paper, and paperboard manufacturers
- Textile mills
- Commercial airports (excludes USDOD facilities)
In addition, the study also attempted to acquire information on the types and concentrations of PFAS discharged in wastewater, as well as assess availability and feasibility of control practices and treatment technologies capable of reducing or eliminating PFAS in wastewater discharges. The information was collected through outreach to stakeholders, including company representatives and trade associations; state, regional, and local wastewater regulatory authorities; and treatment technology vendors. Data sources included 2019 and 2020 NPDES DMRs, USEPA’s Industrial Wastewater Treatment Technology Database, USEPA’s drinking water treatability database, and data made available through other federal agencies such as Department of Transportation, Federal Aviation Administration, Department of Health and Human Services, and Food and Drug Administration, and organizations such as the American Chemistry Council. The report includes detailed information on data sources, and whether any PFAS ELGs have been developed for industries within each of the point source categories.
USEPA Effluent Guidelines Program Plan 15
USEPA discussed its findings from the Multi-Industry PFAS Study and its plans to develop ELGs for PFAS in the 2021 Preliminary Effluent Guidelines Program Plan 15 (USEPA 2021) and the subsequent 2023 Effluent Guidelines Program Plan 15 (Plan 15) (USEPA 2023). A summary is provided below.
OCPSF Manufacturers and Formulators
In December 2021, USEPA used its authority under Section 308 of the CWA to collect data on characterization of wastewater generation, treatment, and discharge from PFAS manufacturing facilities. USEPA “verified that PFAS, including legacy long-chain PFAS and short-chain replacement PFAS, are present in wastewater discharges from OCPSF facilities” (USEPA 2021) to surface waters and POTWs. For both PFAS manufacturers and formulators, average concentrations of short-chain perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkane sulfonic acids (PFSAs) were generally higher relative to long-chain PFCAs and PFSAs. USEPA determined that the development of ELGs for PFAS manufacturers is warranted and plans to revise the existing OCPSF ELGs (40 CFR Part 414). As noted in Program Plan 15, depending on available resources, USEPA intends to publish a proposed rule in spring 2024. In addition, USEPA will continue to evaluate the need to develop regulations to address PFAS discharges from PFAS formulators/processors.
Metal Finishing
USEPA determined that PFAS have, and continue to be, used by metal finishing facilities in the United States. USEPA identified chromium electroplating facilities as the most significant source of PFAS due to their use of PFAS-based mist/fume suppressants to control toxic hexavalent chromium emissions. USEPA determined that the development of effluent guidelines and standards for chromium electroplating facilities is warranted and plans to revise the existing metal finishing ELGs (40 CFR Part 433). Depending on the availability of resources, USEPA intends to collect the data necessary to revise these ELGs, which will include conducting a survey of the industry and analysis of wastewater samples. According to the finalized Program Plan 15, USEPA intends to publish a proposed rule by the end of 2024.
Pulp, Paper, and Paperboard
While USEPA determined that PFAS have been and continue to be used by U.S. pulp, paper, and paperboard facilities, only a small subset of facilities are actively applying PFAS to paper products. The information collected by USEPA indicates that the industry phased out the use of PFOA and PFOS approximately a decade ago but continues to use FDA-approved short-chain PFAS in limited quantities for the manufacture of food contact packaging and specialty paper products. The industry is expected to transition to PFAS-free technologies and eliminate all application of PFAS in U.S. pulp and papermaking operations by 2024. This schedule coincides with an FDA agreement with chemical manufacturers to voluntarily phase out use of PFAS that contain or may degrade to 6:2 fluorotelomer alcohol (6:2 FTOH) in food contact applications by 2024. As a result, a rulemaking on the pulp, paper, and paperboard category is not a priority for USEPA at this time. USEPA plans to continue to study this point-source category, paying particular attention to the potential for legacy discharges after the transition to PFAS-free additives.
Textile and Carpet Manufacturers
As reported in Preliminary Plan 15 (USEPA 2023), much of the information on textile and carpet manufacturers obtained was through a review of publicly available information and literature. Based on a small number of sample results, USEPA determined that PFAS, including legacy long-chain PFAS, are present in wastewater discharges from textile mills to POTWs and that most textile mills were not monitoring for PFAS. Subsequently in November 2021, USEPA used its authority under Section 308 of the CWA to require nine textile manufacturers to provide information on PFAS use and import, PFAS in industrial wastewater discharges, and treatment of PFAS-containing wastewater. Nineteen of 92 (21 percent) reported they used PFAS in 2020, and of those, 18 of the 19 (95 percent) indicated their intention to reduce or eliminate use of PFAS by the end of 2026 by using alternative surface treatment products or technologies. More than half of the textile mills that responded to the survey discharge their process water to a POTW. Two indicated they operate a wastewater treatment system that can effectively remove or eliminate PFAS in wastewater. USEPA plans to expand its study of this category through use of a mandatory, nationally representative questionnaire.
Commercial Airports
USEPA determined that commercial airports may generate PFAS-containing wastewater from live-fire firefighting training, emergency response activities, and accidental leaks from stockpiles of AFFF. However, one outcome of the Federal Aviation Administration (FAA) Reauthorization Act of 2018 was that the FAA has approved and encourages use of different types of AFFF testing equipment that do not require dispensing AFFF when airports conduct periodic equipment testing and training. As of March 2022, the FAA has approved and is funding the use of four different types of firefighting testing devices that do not dispense AFFF and more than half of certified airports have applied these procedures. In addition, as of June 2022, all firefighting foam formulations that meet current military specifications contain less than 800 ppb of PFOS or 800 ppb PFOA. Based on this information, USEPA is not prioritizing rulemaking for this category at this time. However, USEPA will continue to study commercial airport use of AFFF that contains PFAS and will continue to monitor the industry’s transition to fluorine-free foam (see Section 3.11 for more information).
Landfills
Based on public comments to Preliminary Plan 14, USEPA initiated a Landfill Leachate Detailed Study of wastewater discharges from landfills with a focus on PFAS discharges to surface waters and POTWs. Program Plan 15 notes that since September 2021, USEPA has collected data and information on the industry’s facilities, discharge practices, and effectiveness of control practices/technologies to remove PFAS. Depending on available resources, USEPA intends to revise the existing Landfills Point Source Category ELG to address PFAS (schedule yet to be determined).
POTW Influent Study
As noted in Program Plan 15, USEPA intends to collaborate with wastewater treatment facilities to initiate a nationwide study on industrial discharges of PFAS to POTWs. This includes indirect discharges from industrial categories that have been reviewed, as well new categories for which there are very little PFAS data. The goal is to collect samples of PFAS from industrial sources upstream of POTWs (that is, before mixing and dilution from other waste streams). USEPA also intends to develop an Information Collection Request (ICR) and a sampling strategy providing more details about the POTW Influent PFAS Study.
16.7 Surface Water/Groundwater Interaction
As mentioned in Section 16.4.5, surface water/groundwater interaction may play a role in a conceptual site model and locating places to sample. It has been found that PFAS in groundwater that migrates to surface water can be a significant source of PFAS in the surface water, potentially impacting beneficial uses. The same can be said for PFAS in surface water migrating and impacting groundwater quality. These concepts are demonstrated by a case study presented in Section 15.5.2 which describes two examples, one from North Carolina and one from Minnesota. Also see Section 5.3.4.1 for information on the fate and transport of PFAS due to groundwater/surface water interactions and Section 5.3.4.2 for information on surface water/sediment interactions.
Updated September 2023.