9 Site Risk Assessment
The PFAS Team developed a Risk Assessment and Regulations training video with content related to this section. The video provides information on the fundamentals of risk assessment and an overview of the challenges associated with conducting risk assessments for PFAS.
This section discusses the specific challenges associated with assessing and characterizing potential risks to human and ecological receptors exposed to PFAS in the environment. This includes challenges associated with quantifying the degree of exposure, assessing the hazard associated with PFAS, quantifying the dose-response relationship, and characterizing risks to support effective risk management decision-making. Generally, the challenges associated with performing a site risk assessment where the release of PFAS to the environment is suspected are not necessarily unique. Like any other chemical for which there is limited information, knowledge, or other technical complexity, working through the steps necessary to complete a risk assessment would be similar.
Section 15.3 provides a case study example illustrating how the New Jersey Department of Environmental Protection used risk assessment science to help support the development of fish consumption advisories for select water bodies in New Jersey. Section 17.3 provides additional information related to PFAS risk assessment, including (1) exposure pathways relevant for different exposure media, (2) considerations when calculating exposure point concentrations, and (3) selecting bioconcentration/bioaccumulation factors.
| Section Number | Topic |
| 9.1 | Human Health Risk Assessment |
| 9.2 | Ecological Risk Assessment |
| 9.3 | Uncertainty |
9.1 Human Health Risk Assessment
9.1.1 Toxicity Assessment
The toxicity assessment of a site risk assessment involves (1) hazard identification and (2) dose-response assessment. Hazard identification involves determining whether exposures to a chemical can cause an increased risk of an adverse human health effect; dose-response assessment involves quantifying the relationship between the degree of exposure to the chemical and the incidence or severity of the potential adverse effects. More background on each of these steps is detailed in other guidance (USEPA 1989; ITRC 2015) and is not repeated here.
This section discusses specific complications that may be encountered in completing the toxicity assessment for a site risk assessment involving PFAS.
9.1.1.1 Availability of Toxicity Values from a Variety of Sources
A toxicity value (for example, oral cancer slope factor (CSF), or reference dose (RfD)) is a numerical expression of the dose-response relationship for a given substance. It is used in combination with estimates of chemical exposure to calculate quantitative estimates of cancer risk or noncancer hazard (USEPA 1989). Several state, national, and international regulatory and advisory agencies have developed human toxicity values for various PFAS that could be potentially used in conducting risk assessments or in support of establishing policies for PFAS risk management. Given this variety of sources, specific complications can be encountered in determining which toxicity values to use in conducting a risk assessment:
- Selection of toxicity values for PFAS is dependent on which PFAS are present at a given site. PFAS identification and quantification may vary based on analytical method.
- Differences among toxicity values for PFAS could arise because agencies may rely on different toxicity value derivation methods; select critical studies by different criteria, including animal or human data; use different uncertainty factors; and prioritize individual PFAS differently for toxicity value derivation. Table 9-1 provides an example for PFOA and PFOS, showing that USEPA used human data while ATSDR used animal data.
- Available toxicity values may change over time as the results of new studies become available. Newer toxicity values derived by regulatory agencies may be based on more recent and/or different information, methods, and studies than older values, as well as differences in scientific professional judgment and/or different statutory policy requirements. These differences are described in more detail in Section 8.3.
- States may choose to derive their own toxicity values rather than use those developed by the USEPA, and values developed by other countries may differ from USEPA’s.
- Some states may perform route-to-route extrapolation (from oral toxicity values to inhalation toxicity values) for evaluation of the inhalation pathway. However, uncertainties should be noted if using route-to-route extrapolation.
Table 9-1. Example of variability in noncancer toxicity factors for PFOA and PFOS—USEPA (2023),2023 draft RfDs and ATSDR (2021) MRLs.
| Noncancer Toxicity Values for Human Health Risk Assessment (ng/kg body weight*day) |
||||
|---|---|---|---|---|
| Source | PFOA | Basis | PFOS | Basis |
| USEPA Office of Water (2023) DRAFT USEPA (2023) USEPA (2023) Reference Doses (RfDs) |
0.03 | Decreased antibody response to tetanus and diphtheria vaccinations in children, low birth weight, and increased total cholesterol | 0.1 | Low birth weight and increased total cholesterol in humans |
| ATSDR (2021) Minimal Risk Levels (MRLs) |
3 | Behavioral and skeletal effects in mice (following developmental exposure) | 2 | Delayed eye opening and decreased pup weight in rats |
The most recent RfDs available should be used when preparing a human health risk assessment.
There are several options and procedures for selection of toxicity values, as has been described in ITRC guidance (ITRC 2015). For site risk assessments performed in the United States, USEPA, DOD, and other agencies have recommended a tiered hierarchy (Tier 1–Tier 3) of toxicity value sources to guide selection and use (USEPA 2003, 2013); (ECOS-DOD 2007). This recommendation has since been implemented in numerous USEPA OSWER (Currently known as Office of Land and Emergency Management) directives (USEPA 1993, 2003, 2022) that further establish a hierarchy and process for selecting toxicity criteria. For PFAS chemicals as of March 2023:
- Tier 1 values are peer-reviewed toxicity values published on the USEPA’s Integrated Risk Information System (IRIS).
- IRIS values are available for PFBA (USEPA 2022) and PFHxA (USEPA 2023)
- Draft IRIS assessments and toxicity values are available for PFDA (USEPA 2023) and PFHxS (USEPA 2023), and an IRIS assessment and toxicity values for PFNA are under development. The status of the PFAS IRIS assessments can be found in the latest IRIS Program Outlook, available at https://www.epa.gov/iris/iris-program-outlook.
- Tier 2 toxicity values include Provisional Peer-Reviewed Toxicity Values (PPRTV).
- PPRTV values are available for PFBS (USEPA 2021)
- Tier 3 toxicity values include those from additional USEPA and non-USEPA sources. They can include values that may or may not have been peer reviewed. As recommended by USEPA (2003), in using values from Tier 3 sources, it may be appropriate to prioritize those that are the most current, have a transparent basis, are publicly available, have been peer reviewed, and are acceptable to local jurisdictions. USEPA (2022) prioritized available Tier 3 sources of toxicity values in its regional screening level (RSL) guidance, with the current relevant sources for PFAS identified as:
- ATSDR minimal risk levels (MRLs) (for example, the oral MRLs for PFNA and PFHxS (ATSDR 2021))
- USEPA Office of Water health advisories or human health toxicity assessments (for example, the oral toxicity value presented in the drinking water health advisory for GenX [USEPA 2021; USEPA 2022])
- California Environmental Protection Agency Office of Environmental Health Hazard Assessment (CA OEHHA 2023)
- Additional definitions and discussion of PFAS toxicity values that are available for use are provided in Section 7 and Section 17.2.
The USEPA will continue to develop toxicity values for PFAS. In addition, USEPA may continue reviewing available Tier 3 toxicity values and recommending values in RSL table updates. Individual risk assessors can also select Tier 3 values as appropriate (when no Tier 1 or 2 values are available).
9.1.1.2 Characterizing Cancer Risk for Exposure to PFAS
The draft USEPA documents that provide the toxicological basis for the proposed USEPA maximum contaminant level goals (MCLGs) for PFOA and PFOS conclude that both PFOA and PFOS are likely to be carcinogenic to humans (USEPA 2023, 2023, 2023). The draft USEPA MCLGs for PFOA and PFOS are zero, consistent with USEPA’s general approach for MCLGs for known or likely human carcinogens. The International Agency for Research on Cancer (IARC 2016) classified PFOA as possibly carcinogenic to humans (Class 2B). IARC is currently re-evaluating the carcinogenic potential of PFOA and is evaluating the carcinogenic potential of PFOS (IARC 2023). A recent review by NJDWQI (2023) concurred with USEPA that PFOA is a likely human carcinogen; this review also concluded that PFOS has suggestive evidence but was not aware of some animal tumor data relevant to this evaluation that was considered by USEPA (2023) (see Section 17.2.5.3).
For GenX, USEPA concluded that there is suggestive evidence of carcinogenic potential in humans based upon liver, pancreatic, and testicular tumors observed in chronic rat studies, but also concluded that these data lacked a dose-response relationship and therefore did not support development of a CSF (USEPA 2022).
Although most risk-based values and screening levels developed by states have been primarily based on noncancer effects, USEPA and some select states (for example, New Jersey—NJDWQI 2017, 2018; California—OEHHA 2023) have derived oral CSFs for PFOA and PFOS. NJDWQI (2017) developed identical drinking water concentrations for PFOA using the RfD for noncancer effects and the CSF and the 1 in 1 million cancer risk level. For PFOS, NJDWQI (2018) concluded that, although the CSF was too uncertain for use as the quantitative basis to develop a drinking water level, the level based on noncancer effects did not pose an unacceptably high cancer risk. CalEPA’s CA OEHHA (2019), however, issued notification levels for drinking water exposure, and developed draft public health goals (PHGs) for PFOA and PFOS that are driven by carcinogenicity (CA OEHHA 2023). The draft PHG for PFOA is based on kidney cancer in humans; the PHG for PFOS is based on liver and pancreatic tumors in rats (CA OEHHA 2023). As part of the basis for the proposed USEPA MCLs for PFOA and PFOS, USEPA (2023; 2023; 2023) developed a draft CSF for PFOA of 0.0293 (ng/kg/day)-1 (i.e., 29,300 [mg/kg/day]-1) based on kidney cancer in the human general population and a draft CSF for PFOS of 39.5 (mg/kg/day)-1 based on liver tumors in rats. For site risk assessments, the derived CSFs developed by these agencies could be used.
Further discussion of the carcinogenicity of PFAS is presented in Section 17.2.4.2 (Carcinogenicity), Section 17.2.5.3 (Chronic Toxicity and Tumorigenicity), and Section 8.2.2.6 (CERCLA).
9.1.1.3 Lack of Toxicological Values for Many PFAS
There are several thousand PFAS that could have been, or may be, on the global market (OECD 2018), although the uses of all of these PFAS may not be known (KEMI 2015). More information about PFAS in use is included in Section 2. In general, PFAS are considered to be bioavailable (i.e., following exposure, they are absorbed and enter the systemic circulation). However, toxicity values have been developed for only a few PFAS for which sufficient information is available. Because of the lack of hazard and dose-response information for other PFAS and the extensive level of effort needed to develop toxicity values, there are no readily available toxicity values for the majority of PFAS.
This lack of information precludes the establishment of compound-specific risk-based concentrations that can be helpful for a variety of applications, including data screening (used to help guide site investigation) and site-cleanup decision-making. In the absence of toxicity values, regulatory agencies and the regulated community are left with uncertainty regarding the potential risks associated with human exposure to impacted environmental media at sites, difficulties with creation of technically defensible risk management programs, and inability of the regulated community to be responsive to concerns about environmental risk.
An approach often used in HHRA in the absence of compound-specific toxicity values is to use toxicity values developed for structurally or chemically similar surrogate compounds with similar biological activity. In the case of PFAS, this would be for PFAS from the same structural subgroup (for example, long-chain perfluorocarboxylic acids). The use of surrogates, however, introduces uncertainty, because surrogates may produce adverse health effects by mechanisms different from the compound of concern, the dose-response curve for a surrogate may be different, and the target organ or toxicity endpoint may be different from the compound of concern. In the absence of chemical-specific toxicity values, preparation of health risk assessments may be limited to qualitative methods and have a higher level of uncertainty in the human health risk assessment as a result.
Further information and guidance are needed to identify appropriate surrogates for PFAS that do not currently have available toxicity values. USEPA’s PFAS Action Plan (USEPA 2019) noted that USEPA would be working on developing an approach to PFAS toxicity testing that could lead to a methodology for inferring the toxicology of a given PFAS based on the toxicology of a PFAS subset whose toxicology is known. This involves “applying computational and high throughput toxicology tools for PFAS toxicity testing on a larger scale to enable faster understanding of potential toxicity for the universe of thousands of PFAS, most of which have little or no published toxicity data” (USEPA 2022). A more thorough discussion of new assessment methods (NAMs) can be found in Section 17.2.7.
The USEPA has developed its National PFAS Testing Strategy in which PFAS manufacturers will be issued orders under Toxic Substances Control Act (TSCA) authorities to perform toxicity testing on compounds representative of certain PFAS classes (USEPA 2021). These classes are organized by similarities in structure, physical-chemical properties, and existing test data on compound toxicity. Thus, there is a plan to obtain toxicity information for each class via a representative compound that will potentially serve as a surrogate for the other compounds in the class.
9.1.2 Exposure Assessment
The exposure assessment of a site risk assessment involves characterizing the exposure setting, identifying relevant exposure pathways and scenarios, and quantifying the magnitude, frequency, and duration of potential human exposure to chemicals in environmental media. More background on the performance of exposure assessments is detailed in other guidance (USEPA 1989; ITRC 2015) and is not repeated here.
This section discusses specific complications that may be encountered in completing the exposure assessment for a site risk assessment involving PFAS. It should be recognized that the exposure assessment does not generally account for the presence of all PFAS at a site due to limitations in analytical methods. Therefore, there are uncertainties in the characterization of exposures (and associated risks) at PFAS sites that should be acknowledged in the uncertainty analysis section of the risk assessment.
PFAS may be present in biosolids at levels of potential concern. If biosolids contain PFAS and are applied to agricultural fields, the PFAS may contaminate crops and livestock. Blaine et al. (2013) evaluated the uptake of PFAAs contained in industrially impacted biosolids-amended soil and municipal biosolids-amended soil by various crops and confirmed “that the bioaccumulation of PFAAs from biosolids-amended soils depends strongly on PFAA concentrations, soil properties, the type of crop, and analyte.” In addition, Lindstrom et al. (2011) evaluated the impacts of PFAS-contaminated biosolids from a local municipal wastewater treatment facility (that historically received waste from fluorochemical facilities) used as a soil amendment in local agricultural fields. Results showed relatively high transport from soils to surface water and groundwater in the vicinity of the agricultural fields. Based on these studies, two potential exposure pathways to PFAS in biosolids are drinking water ingestion and food ingestion. A discussion of the leaching potential from soil to groundwater for biosolids is provided in Section 6.2.3. As indicated in Section 6.2, a 2021 review of reported values for biosolids, compost, and related biowastes highlights the wide concentration range of reported PFAS (primarily PFAAs) and the relationship of biowaste source to resulting concentrations (Bolan et al. 2021).
Biosolids as a potential source of PFAS are mentioned in USEPA’s (2021) PFAS Strategic Roadmap. USEPA is currently conducting a risk assessment for PFOA and PFOS in biosolids and expects to have the risk assessment finalized in 2024.
9.1.2.1 Determining Scenarios for Potential Human Exposure
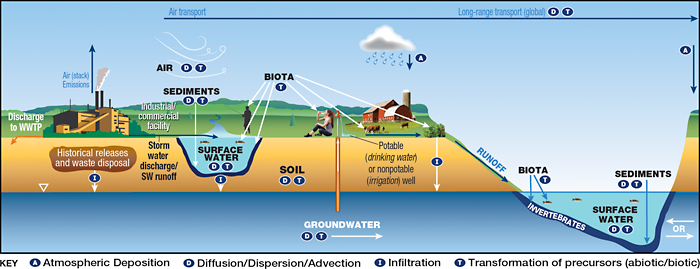
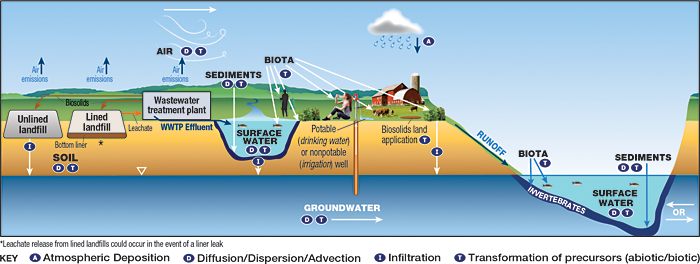
A site-specific conceptual exposure model should be developed during the planning stage of the HHRA, confirmed by stakeholders, and updated as additional information and data are obtained, (see Section 3 of the RISK-3 guidance (ITRC 2015)). The specific exposure scenarios that are applicable to an HHRA for PFAS include those that could occur in media at the release area (the site) and in media at distant locations (with the extent depending on PFAS properties and the site setting). In general, an HHRA for PFAS may be complex in comparison to HHRAs for other types of chemicals due to the persistence of PFAS, the complexities associated with PFAS toxicity, and complexities associated with estimating future concentrations or modeling their fate and transport, and the need to include more media than is typical. Figures 9-1, 9-2 and 9-3 are provided below to illustrate conceptual site models (CSMs) for four sources (two sources are illustrated in Figure 9-3) of PFAS. Section 2.6 discusses potential environmental releases of PFAS. A detailed discussion of fate and transport processes for PFAS and environmental media that may be affected is presented in Section 5.
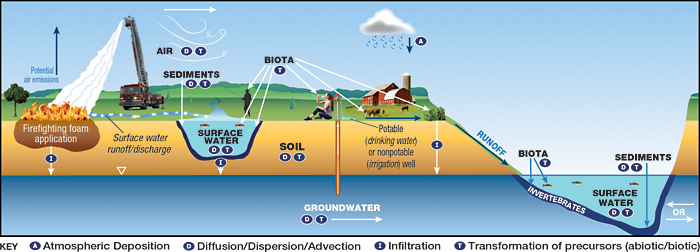
Figure 9-1. CSM for fire training area.
Source: Adapted from figure by L. Trozzolo, TRC. Used with permission.
Figure 9-2. CSM for industrial sites.
Source: Adapted from figure by L. Trozzolo, TRC. Used with permission.
Figure 9-3. CSM for landfills and WWTPs.
Source: Adapted from figure by L. Trozzolo, TRC. Used with permission.
Various exposure scenarios may be possible for a given site. Specific exposure scenarios that could be included in an HHRA are a site-specific decision.
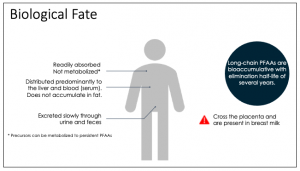
The highest exposures to PFAS can occur during early life stages (Goeden, Greene, and Jacobus 2019). Exposures to infants from breast milk of exposed mothers (Figure 9-4) or formula prepared with contaminated water are higher (on a body weight basis) than in older age groups (Fromme et al. 2009; Mogensen et al. 2015; Verner et al. 2016, Verner et al. 2016; Post, Cohn, and Cooper 2012; Goeden, Greene, and Jacobus 2019). The higher exposures during pregnancy and to infants are of concern because fetuses and infants are potentially sensitive subpopulations for developmental effects of some PFAS, including PFOA and PFOS (USEPA 2023, USEPA 2023), as discussed in Section 7.1. Therefore, exposure scenarios that include fetuses, infants, children, adolescents, and women of childbearing years should be considered in HHRAs.
Figure 9-4. Biological fate of long-chain PFAAs.
Figure 9-5 illustrates the predominant exposure pathways. More detailed information about these exposure pathways, as well as other environmental medium-specific issues affecting potential human exposure scenarios, are provided in Section 17.3.1.
Figure 9-5. Predominant human exposure pathways.
9.1.2.2 Calculating Exposure Concentrations for PFAS via Fate and Transport Models
When using fate and transport models to calculate exposure point concentrations (EPCs) for PFAS, it is important to note that individual PFAS have different chemical properties that affect their fate in the environment (Section 5). Some PFAS are mobile, persistent, and bioaccumulative (in wildlife and humans), and others are not. Perfluoroalkyl acids (PFAAs) are persistent, and long-chain PFAAs bioaccumulate in humans (USEPA 2003; ATSDR 2020; NTP 2016; CONCAWE 2016). USEPA has compiled an online resource for PFAS information that includes guidance on environmental behavior and site characterization (USEPA 2017). The National Groundwater Association (NGWA) has also published a resource on PFAS that includes information about fate and transport (NGWA 2017). Section 5.2.3 provides a discussion of fate and transport modeling for PFAS, including numerous citations. Additional information is included in Section 17.3.2.
When using environmental fate and transport models for estimating EPCs in biota, modeling should be focused on the part of the organism that may be consumed either by humans or by ecological receptors, recognizing that patterns of consumption may be influenced by a number of socioeconomic and cultural factors. PFAS generally bind to proteins and accumulate in protein-rich tissues, including the blood, liver, and kidneys (ATSDR 2020). Plant uptake and bioaccumulation and partitioning within the plant appear to depend on PFAS chemical structure and the plant species (see Section 5.6). Section 17.3.3 includes information about selecting bioaccumulation and bioconcentration factor values.
Measured concentrations at exposure points may differ from modeled EPCs. This may be due to other sources of PFAS (for example, a nearby site that had a PFAS release to the ground and that subsequently leached to groundwater) also contributing to concentrations at the exposure point and the limitations of the models currently available.
In surface water bodies, PFAS concentrations in foam formed at the air-water interface from wind or wave action may be much higher than PFAS concentrations present in the water column (see Section 16.5). Therefore, in surface water bodies where foam is present, the need to evaluate exposures to foam should be considered when planning an HHRA. At sites where foam exposures are evaluated in the HHRA, EPCs should be established separately for foam and surface water so that potential risks to foam are not underestimated.
9.1.3 Risk Characterization
The risk characterization of a site risk assessment combines the results of the exposure assessment and the toxicity assessment to provide a quantitative estimate of risk (ITRC 2015). It also may include a qualitative narrative designed to provide decision makers with information regarding key assumptions, uncertainties, or other issues that would be important to understand when making risk management decisions. More background on the performance of risk characterizations is detailed in other guidance (USEPA 1989; ITRC 2015) and is not repeated here.
Because risk characterization involves combining the toxicity assessment and exposure assessment, the complexities discussed in Sections 9.1.1 and 9.1.2 manifest themselves in the risk characterization. There are, however, additional specific complications that may be encountered in completing the risk characterization for a site risk assessment involving PFAS. This section discusses those specific complexities.
9.1.3.1 Assessing the Cumulative Effects of Exposure to PFAS
The overall potential for noncancer effects due to human exposure to more than one chemical is estimated using the hazard index (HI), which is computed as the sum of calculated chemical-specific hazard quotients (HQ). As explained by USEPA (1989), “This approach assumes that simultaneous subthreshold exposures to several chemicals could result in an adverse effect. It also assumes that the magnitude of the adverse effect will be proportional to the sum of the ratios of subthreshold exposures to acceptable exposures.” Risk characterizations commonly produce initial estimates of HI by calculating the sum of all HQs. When the HI is estimated to be greater than 1, there may be potential concern for adverse health effects. However, when this initially estimated HI is greater than 1, refinement of the HI estimate by segregating HIs by effect and mechanism of action may be appropriate to support a risk management decision.
USEPA has developed a Draft Framework for Estimating Noncancer Health Risks Associated with Mixtures of Per- and Polyfluoroalkyl Substances (PFAS) (USEPA 2023). This document recommends assessment of noncancer health risks of PFAS mixtures based on dose additivity (for example, a hazard index [HI] approach). USEPA (2023) is the basis for a proposed MCLG and MCL of an HI of 1 for mixtures of four PFAS (PFBS, PFHxS, PFNA, GenX) (USEPA 2023). The draft MCLG and MCL use the “general” HI approach in which the HI considers toxicity values based on differing toxicological endpoints for the components of the mixture. More information on risk assessment of PFAS mixtures is found in Section 7.1.5.
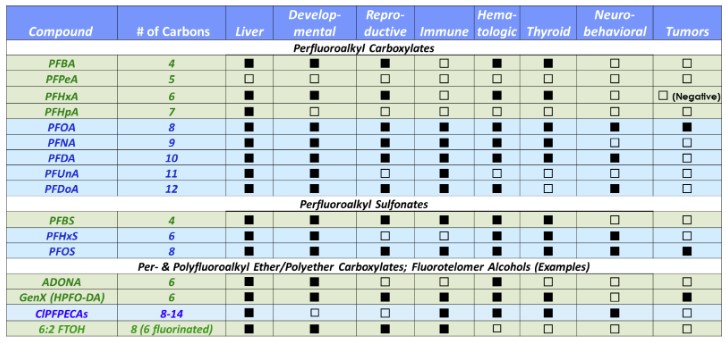
As discussed in Section 7.1, several possible adverse health effects are associated with exposure to PFAS (Table 9-2). The type of information shown in this table can be used to segregate HIs by potential adverse effect in the risk characterization (i.e., the “Target Organ-Specific Hazard Index”) when risks associated with exposure to specific PFAS are being evaluated and target organ-specific toxicity factors are available.
Table 9-2. Summary of potential noncancer health effects of various PFAS
Adapted from (ATSDR 2022)
Notes:
The colors used for the PFAS names distinguish between short-chain PFAS (green) and long-chain PFAS (blue).
A filled-in (black) box indicates that the effect was reported in one or more laboratory animal studies. An empty box indicates that the effect was evaluated but not found, or effect has not been evaluated.
9.2 Ecological Risk Assessment
This section summarizes information that is currently available to conduct ecological risk assessment (ERA) of PFAS. The information presented is based on a review of current regulatory guidelines from within the United States and other jurisdictions, peer-reviewed publications, and other sources as noted. Sufficient information needed for performing ERAs exists only for some PFAS. When possible, discussions in this section indicate to which PFAS the discussions apply. Three recent publications funded by the US DOD, Conder et al. (2020), Divine et al. (2020), and Argonne (2021) discuss PFAS ERA in detail and provide a summary of much of the available data needed for conducting PFAS ERA within the U.S. These are the most comprehensive reports to date for completing PFAS ERAs within the U.S. The European Union (EU) also has a substantial amount of data available within their Environmental Quality Standards (EQS) for performing PFAS ERA, but the EQS use different guidelines and approaches than those in the U.S. Use of these data will likely result in conflicting values and differing conclusions on data usability and adequacy for performing an ERA.
This section discusses challenges within three key components of ERA: ecological effects assessment, exposure assessment, and risk characterization. Conder et al. (2020) and Divine et al. (2020) are referenced throughout those discussions as applicable. In addition, a workshop sponsored by the Society of Environmental Toxicology and Chemistry (SETAC) was held in August 2019 and focused on the state-of-the-science supporting risk assessment of PFAS. A breakout group within that workshop focused specifically on ecotoxicology and ecological risks of PFAS. The expert panel in that breakout group produced a detailed manuscript describing the currently available information, data gaps, and uncertainties, and approaches to address these needs (Ankley et al. 2020). Like the Conder et al. (2020) and Divine et al. (2020) reports, the recommendations of Ankley et al. (2020) are also directly related to conducting PFAS ERAs and are mentioned here to provide readers an understanding of subjects that are relevant but that still have uncertainty so they can assess the importance of those subjects for their specific needs. Ankley et al. (2020) is referenced throughout this section as applicable. Additional recommendations from Ankley et al. (2020) not included later in this section are listed below:
- a need for prioritizing which PFAS to study and evaluate relative to ecological risk and toxicity
- environmental monitoring beyond PFOS and PFOA
- advancing the understanding of PFAS uptake, elimination, and bioaccumulation
- broader understanding of toxicity across taxa
- the use of new approach methods (often referred to as NAMs) and
- studying and assessing PFAS as mixtures.
The remaining text for this section is organized into three main components of ERA: ecological effects assessment, exposure assessment, and risk characterization.
9.2.1 Ecological Effects Assessment
Identification of ecological risk-based toxicity thresholds is a challenge for many PFAS. Toxicity data are available as discussed in Section 7.2. Some of these data have been used to establish thresholds as discussed below. Some major considerations for ecological effects assessment are identifying ecological screening thresholds, understanding ecological receptor variability, and evaluating ecological toxicity of mixtures. These are discussed in the following sections.
9.2.1.1 Ecological Screening Thresholds
The recently published manuscript by Ankley et al. (2020) includes a discussion of media-specific ecological screening thresholds that are available for certain PFAS around the world: PFOS, PFOA, PFBA, PFPeA, PFHxA, PFBS, and PFHxS. Ankley et al. (2020) did not claim the list of thresholds to be comprehensive of all ecological thresholds for PFAS around the world. The discussion here in this section presents thresholds that are included within that document with discussion relevant to ITRC’s intended audience. Ankley et al. (2020) can be referenced for a more detailed discussion and complete table of available thresholds from around the world.
U.S.—Federal Thresholds
USEPA has published draft national recommended aquatic life criteria for PFOA and PFOS in freshwater for public comment (USEPA 2022, 2022) and a fact sheet for the criteria (USEPA 2022). In addition, USEPA had published their responses to external peer reviews of the draft criteria (USEPA 2022, 2022). The draft criteria include acute and chronic water column values for freshwater environments and acute criteria for estuarine/marine environments. Tissue criteria are also proposed for whole body fish and benthic invertebrates and for fish muscle. These criteria are aimed at protection of aquatic life but are not protective of consumption by aquatic dependent wildlife (for example, birds and mammals foraging in aquatic environments). Currently, there are no ecological risk-based PFAS guidelines or media screening thresholds that are recommended by the USEPA for other PFAS.
In support of the Air Force Civil Engineer Center, Argonne National Laboratory developed ecological screening thresholds for surface water and soil (Argonne 2021). Values were developed for 4-, 6-, 8-, 9-, and 10-carbon linear PFCAs and the 4-, 6-, and 8-carbon linear PFSAs (see Section 2 for detailed naming conventions). The soil screening values are protective of terrestrial plants, invertebrates, and mammalian and avian wildlife. The surface water values are protective of aquatic life and aquatic dependent wildlife. Work was completed in consultation with Tri-Services Environmental Risk Assessment Working Group, and USEPA’s Ecological Risk Assessment Forum (Ankley et al. 2020).
There have also been a number of published studies and reports that follow U.S. federal guidelines (USEPA 1995) for developing aquatic life criteria. Giesy et al. (2010), Salice et al. (2018); Conder et al. (2020), and Divine et al. (2020) all calculated thresholds protective of aquatic organisms exposed to PFOS in freshwater environments, though these were published before USEPA released their draft criteria. Giesy et al. (2010) also reported a Tier II freshwater value for PFBS, while Conder et al. (2020) derived Tier I values for PFOS in both freshwater and marine environments and for PFOA for freshwater. Divine et al. (2020) developed Tier I freshwater values for PFOS and PFOA and Tier II values for 23 other PFAS. Tier I values meet the data required described in USEPA (1995) guidelines while Tier II values are developed with methods that incorporate uncertainty factors when the availability of required data is insufficient. Section 16.3 provides greater detailed discussion on the methods for developing thresholds protective of aquatic life.
U.S.—Thresholds for Specific States
Several states have established some criteria that are intended to protect aquatic organisms in their respective surface waters. The text below is not intended to be exhaustive and summarizes only some of the values that were available at the time this text was developed (Spring 2021).
In Michigan, AWQC have been established for PFOS and PFOA based on Rule 57 17 (MI EGLE 2019). This rule is based on the USEPA Great Lakes Initiative (USEPA 1995), which provides procedures and methodologies to derive numerical criteria that are protective of aquatic ecosystems. Rule 57 presents a two-tiered methodology in which Tier I procedures are essentially the same as the methods used to derive federal national water quality criteria (NWQC) (USEPA 1985) and Tier II procedures can be used to derive values where the full extent of the toxicity data requirements of NWQC are not fulfilled. Rule 57 presents procedures to develop three categories of numeric criteria—final chronic values (FCVs), aquatic maximum values (AMVs), and final acute values (FAVs)—which can be developed under either Tier I or Tier II. Due to the greater uncertainties associated with Tier II values, and given their lesser data requirements, these values tend to be more conservative than those derived with Tier I methodologies. The PFOA and PFOS numeric criteria for Michigan are all Tier II values due to the limited amount of peer-reviewed aquatic toxicity data. The final chronic values for the protection of aquatic life (flora and fauna) for PFOA and PFOS were 880 and 140 µg/L, respectively, while aquatic maximum values were 7,700 and 780 µg/L, respectively. In addition, the Michigan Department of Community Health (MDCH 2015) derived provisional PFOS surface water values for mammalian and avian wildlife based on Rule 57 guidance. The surface water avian wildlife value, based on eagles, kingfishers, and herring gull characteristics, was 0.035 µg PFOS/L. The mammalian wildlife value, based on otter and mink characteristics, was 0.084 µg PFOS/L.
The State of Minnesota has also derived several surface water criteria for the protection of aquatic biota. These values are based on guidelines in Minnesota Rules chapter 7050 (MR7050). Continuous chronic criteria for the protection of aquatic biota in surface water are available for PFOA (1,700 µg/L) and PFOS (19 µg/L) (Stevens and Coryell 2007; Stevens and Coryell 2007). Florida has established Provisional Surface Water Screening Levels that are in the same range as those for Minnesota: 1,300 µg/L PFOA in freshwater, 37 µg/L PFOS in freshwater, and 13 µg/L PFOS in salt water (FL DEP 2020).
In California, the San Francisco Bay Regional Water Quality Control Board (SFB RWQCB) has released Interim Final Environmental Screening Levels (ESLs) for PFOS and PFOA (SFB RWQCB 2020). These values were specifically developed for use within the jurisdiction of the specific water board, not the state of California, not the California Department of Toxic Substances Control, and not the entirety of the United States. Other jurisdictions may use these values, as is often the case with many published thresholds. However, with the number of different water boards in California, it is important to understand the applicability of these ESLs. These values include groundwater protection levels that are protective for direct exposure to freshwater and marine organisms, including rare, threatened, and endangered species. The values are based on a 99% protection level (lower 1st percentile of a species sensitivity distribution [SSD]) compared to a 95% protection level (lower 5th percentile of an SSD) used for a typical AWQC. There are also groundwater ESLs that are protective of adverse effects to birds and mammals from the consumption of aquatic prey (Table 9-3). These wildlife protection values (listed by the SFB RWQCB and in Table 9-3 as secondary poisoning ecotoxicity) are based on values published in Divine et al. (2020). Separate soil ESLs are included in the SFB RWQCB document that are protective of (1) plants and invertebrates or (2) birds and mammals. There are two soil ESLs for each chemical for both significantly vegetated and minimally vegetated areas for a total of four distinct values each for PFOS and PFOA as shown in Table 9-4.
Table 9-3. SFB RWQCB groundwater ESLs: Aquatic habitat ecotoxicity levels for PFOS and PFOA (SFB RWQCB 2020)
| Protected Organisms | PFOS (µg/L) | PFOA (µg/L) |
|---|---|---|
| Direct exposure ecotoxicity: Freshwater | 0.56 | 540 |
| Direct exposure ecotoxicity: Saltwater | 2.6 | 540 |
| Secondary poisoning ecotoxicity: Freshwater and saltwater | 0.075 | 4.4 |
Table 9-4. Soil ESLs: Terrestrial habitat levels (SFB RWQCB 2020)
| Protected Organisms | PFOS (mg/kg) | PFOA (mg/kg) |
|---|---|---|
| Significantly vegetated areas | ||
| Plants and invertebrates | 7.7 | 0.084 |
| Mammals and birds (NOAEL-based) | 0.013 | 0.57 |
| Minimally vegetated areas | ||
| Plants and invertebrates | 33 | 0.84 |
| Mammals and birds (LOAEL-based) | 0.05 | 1.1 |
International Thresholds
Environment and Climate Change Canada (ECCC, previously known as Environment Canada) has proposed ecological Federal Environmental Quality Guidelines (FEQGs) for PFOS in surface waters, fish tissue, wildlife dietary values, and bird eggs (ECCC 2018). The PFOS threshold for surface waters was derived from a SSD based on long-term toxicity data that included data for amphibians, fish, invertebrates, phytoplankton, and macrophytes. The guideline to protect all aquatic life forms for indefinite exposure periods to PFOS in surface waters is 6.8 µg/L, and a whole-body fish tissue guideline value of 9.4 mg/kg wet weight (ww) was based on these fish data and bioaccumulation factors for bluegill from Drottar, Van Hoven, and Kruger (2002). The tissue threshold is intended for both freshwater and marine environments. It was not calculated with both food and water (direct media) BAFs, and thus it could be underprotective. However, Giesy et al. (2010) did use Drottar, Van Hoven, and Kruger (2002) data to calculate an acute no-effect threshold of 87 mg/kg ww whole-body fish. To protect mammalian and avian consumers of aquatic biota, ECCC derived wildlife dietary toxicity reference values (TRVs) using mammalian studies and avian chronic toxicity data. For mammals, the dietary value for PFOS was 4.6 µg/kg ww food while the avian dietary value was 8.2 µg/kg ww food. Based on the avian reproduction studies that were the basis for the dietary values, a guideline of 1.9 µg/g ww whole egg was also derived for PFOS.
Screening level assessment values have also been derived for PFOA (Environment Canada 2012). Environment Canada derived several predicted no-effect concentrations (PNECs) for PFOA for ecological species. PNECs are intentionally conservative concentrations of chemicals designed to represent a concentration at which no adverse effects are likely. These PNECs for PFOA were based on LOAEL values from a limited set of single organism toxicity studies adjusted with uncertainty factors. FEQG values are developed from a distribution of acute and chronic studies conducted on groups of organisms with an intent to be protective of a set percentage of organisms in that category (for example, a 95% protection threshold). Thus, these PFOA PNECs are not equivalent to FEQGs, though they still provide utility for screening level ERA. The PNEC for aquatic organisms, based on a study with the freshwater alga Pseudokirchneriella subcapitata, was 20 µg/L; a mammalian wildlife study based on cynomolgus monkey (Macaca fascicularis) derived a liver-based PFOA PNEC of 158 µg/kg ww. However, given the uncertainties associated with these values, care should be taken in their application to ERA. FEQGs for PFOA are currently under development by ECCC (ECCC 2018).
The Australian and the New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand (ANZECC/ARMCANZ) have established draft protective concentrations for freshwater organisms exposed to PFOS and PFOA. The values, as shown in Table 9-5, were developed by the Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC CARE) (2018). Only the freshwater values have been adopted in the PFAS National Environmental Management Plan for Australia and New Zealand (HEPA 2020). The protocol for developing guideline values in Australia (Warne et al. 2018) includes some specifications for full lifecycle and multigenerational testing that were not sufficiently represented in the limited available marine studies. Instead, the freshwater values have been identified as interim thresholds for marine waters. The values from CRC CARE incorporated multiple studies and were based on SSD for each compound. The 90 and 95% protective thresholds for PFOS are 2.0 and 0.13 µg/L, respectively. These values are within the range of other published values (Giesy et al. 2010; Qi et al. 2011). A 99% protection value for PFOS was also proposed as 0.00023 µg/L, though this may be below ambient concentrations. It should be noted that the freshwater SSD and subsequent protection values are influenced by a multigenerational study (Keiter et al. 2012) with zebra fish (Danio rerio) that produced data that are noticeably lower than other data. Efforts to validate the data by repeating the study and its methods are ongoing at the US Army Corps of Engineers Environmental Research and Development Center (Gust et al. 2021). The ongoing work includes an expanded and more carefully determined dosing range, greatly increased replication base on statistical power analysis, expanded analytical chemistry with external validation, and an expanded suite of endpoints to improve overall toxicological context (Gust et al. 2021). All three of the PFOS protection values are taken from an SSD that includes studies on the low end that are well below the majority of other data points. Further, as indicated in table B3 of CRC CARE (2018), data used in the SSD include a mix of effect levels (EC10) and no-effect levels (NOECs). Thus, decisions based on these values should be made with careful consideration.
For PFOA, the 90 and 95% protective thresholds were 632 and 220 µg/L, respectively; these are similar to those derived in Minnesota and Michigan. Marine threshold values for PFOS were 32 and 7.8 µg/L for the 90 and 95% protective levels. For PFOA, the 90 and 95% protective thresholds for marine water were 14,000 and 8,500 µg/L, respectively. It is of note that the threshold values for marine species were at least 1–2 orders of magnitude greater than those from freshwater. Thus, one should take care in using freshwater toxicity data or threshold values when evaluating marine and brackish systems, given the apparent differences in species sensitivity between these two environments. Likewise, caution should be used if employing marine values to evaluate other PFAS for which there are no freshwater threshold values.
Table 9-5. Aquatic thresholds developed by CRC CARE (CRC CARE 2018)
| Species Protection (%) | PFOS (μg/L) | PFOA (μg/L) |
|---|---|---|
| Freshwater | ||
| 80 | 31 | 1,824 |
| 90 | 2 | 632 |
| 95 | 0.13 | 220 |
| 99 | 0.00023 | 19 |
| Marine | ||
| 80 | 130 | 22 |
| 90 | 32 | 14 |
| 95 | 7.8 | 8.5 |
| 99 | 0.29 | 3 |
A number of thresholds for PFOS are also available from the European Union (EU) as described in the Environmental Quality Standards Dossier (EQS) for PFOS (European Union 2011, 2013). These include maximum acceptable quality standards (MAC-EQS) for freshwater and marine ecosystems, and annual average quality standards (AA-EQS) for the same ecosystems. Standards are also available for secondary poisoning (that is, consideration of biomagnification through the consumption of contaminated prey). These values are shown in Table 9-6.
Table 9-6. Environmental quality standards (EQS) for PFOS (European Union 2011, 2013)
| Category/Description | Units | Value |
|---|---|---|
| MAC-EQS (freshwater) (European Commission 2011, 2013) | μg/L | 36 |
| MAC-EQS (marine) (European Commission 2011, 2013) | μg/L | 7.2 |
| Pelagic Community EQS (freshwater) (European Commission 2011) | μg/L | 0.23 |
| Pelagic Community EQS (marine) (European Commission 2011) | μg/L | 0.023 |
| QSbiota, sec pois (European Commission 2011) | mg/kg (ww) | 0.033 |
| QSbiota, sec pois (freshwater) (European Commission 2011) |
μg/L | 0.002 |
| QSbiota, sec pois (marine) (European Commission 2011) |
μg/L | 0.00047 |
| EQS (biota) (European Commission 2011, 2013) | μg/L | 9.1 |
| AA-EQS (freshwater) (European Commission 2011, 2013) | μg/L | 0.00065 |
| AA-EQS (marine) (European Commission 2011, 2013) | μg/L | 0.00013 |
| MAC-EQS = maximum acceptable environmental quality standard AA-EQS = annual average environmental quality standard QS biota, sec pois = secondary poison standard for concentration in fish tissue QS = quality standard EQS (biota) – environmental quality standard |
||
Other Considerations for Thresholds
A survey of reports from various regulatory agencies demonstrates that although ecotoxicity data are available for various PFAS, including PFBA, PFBS, and fluorotelomers (including 8:2 FTCA, 8:2 FTUCA, and several FTOHs), to date these typically consider only a few aquatic species that include D. magna, a green alga, and perhaps a fish species. Some regulatory programs (for example, USEPA 1985; Warne et al. 2018) require a robust data set covering several classes of organisms, and due to limitations in the number of classes of organisms represented in the published peer reviewed data, it is difficult to derive ambient surface water quality criteria for other PFAS (beyond PFOS and PFOA). Limited relevant toxicity data is a greater issue for terrestrial wildlife given that the only chronic, reproductive studies with two PFAS (PFOS and PFOA) that have been conducted to date are in two species, the bobwhite quail (Colinus virginianus) and mallard duck (Anas platyrhynchos). However, more data are becoming available for other PFAS (for example PFHxS and PFHxA) in avian laboratory models (Dennis et al. 2021). Importantly, no ecologically relevant studies have been conducted with mink or an adequate surrogate. As a result, the development of benchmark or threshold concentrations for wildlife and aquatic organisms has been slow but information is beginning to emerge.
Research on observed effects in benthic invertebrates and other benthic aquatic life with direct exposure to sediments contaminated with PFAS is limited. However, more recent literature is becoming available for sediment exposures (Marziali et al. 2019), there are no published benchmarks and publications are few. Research has focused more on aqueous exposure pathways. Observational data and monitoring have been used in some cases to develop an understanding of what exposure may be associated with effects. The Norwegian Pollution Control Agency (NPCA) established a sediment no-effect threshold of 220 µg/kg, a chronic toxicity range of 220–630 µg/kg, and an acute short-term effects range of 630–3,100 µg/kg (NPCA 2008; Bakke et al. 2010) for benthic invertebrates. The technical basis for the NPCA values relied on the principles of equilibrium partitioning (EqP) between sediment and surface water using a partitioning coefficient (Kd). Argonne (2021) also outlines this approach for determining site-specific sediment thresholds. The NPCA no-effect sediment value is based on a PNEC of 72 µg/L from a limited data set and an unspecified Kd. A sediment screening value of 6.7 µg/kg (wet weight) from the United Kingdom’s UK Environment Agency (2004) was also developed using EqP with a PNEC of 2.5 µg/L and a river sediment Kd of 8.7 L/kg. Simpson et al. (2021) have estimated sediment thresholds of 60 µg/kg PFOS and 250 µg/kg PFOS (both normalized to 1% organic carbon) protective of 99% and 95% of species, respectively, based on EqP using a Kd of 10 L/kg with an HC1 of 6 µg/L and HC5 of 25 µg/L. HC5 and HC1 are commonly established for contaminants from SSDs developed for the contaminant. These HCx (for example a HC5) represent the concentration above which the lower x proportion of species in the distribution may see adverse effects.
All of these sediment thresholds were reported for PFOS concentrations in marine sediments, though they provide some basis for screening level risk decisions for both marine and freshwater. Caution should be observed in using these values because associated effects, if any, are unclear, and the original work is not readily available. Caution should also be used in applying these NPCA sediment values from marine waters to freshwater because the freshwater organisms could be more exposed (as explained in Section 9.1.2) and either more or less sensitive than marine organisms. In its EQSD for PFOS, the European Union (2011) took the position that there is insufficient data available to confirm the need for a sediment quality standard and insufficient data to derive a threshold, thus electing not to develop a value. Similarly, a workgroup in northern Italy concluded that there was no need for a sediment environmental quality standard (EQS) for PFOA, PFBS, PFBA, and PFPeA and that data for a sediment EQS for PFHxA were insufficient (Valsecchi et al. 2017).
For soil, CRC CARE developed soil screening thresholds from SSDs for both PFOS and PFOA. The Canadian Council of Ministers of Environment (CCME 2018) has also developed several draft thresholds for PFOS in soil. A value protective of direct toxicity was developed from an SSD of plant and invertebrate IC25 values (the concentration at which a 25% reduction in a non-lethal biological measurement, such as growth or reproduction, occurs). Food chain models were used to develop values protective of soil and food ingestion by wildlife. CCME (2018) also developed a soil screening value protective of aquatic life for use at sites where off-site migration to nearby surface water bodies may be a concern. These values from CCME were issued draft for public comment, and final FEQGs have not yet been established. Soil threshold values for other PFAS, however, are limited.
9.2.1.2 Ecological Receptor Variability
Another major challenge with toxicity assessment for ERA is accounting for the large number of receptor types and the associated unknown variable sensitivity to PFAS. Although it is commonly understood that sensitivity to contaminants can vary widely across kingdoms or across classes of animals, the challenge for PFAS may be greater due to the lack of knowledge about this family of compounds. Studies have documented the presence of PFAS in various aquatic species since the 1950s (Danish EPA 2015; Giesy and Kannan 2001, 2002), such as bottle-nosed dolphins (Houde et al. 2006), seals (Butt et al. 2008), squid (Yang et al. 2012), alligators (Bangma et al. 2017), and polar bears (Smithwick et al. 2005; Smithwick et al. 2005; Greaves and Letcher 2013). The detection of PFAS within organisms is clear evidence of exposure. Unlike many other commonly detected contaminants, however, the availability of toxicological data for PFAS is limited relative to the broad range of organisms within which PFAS have been detected.
Standard ERA practice includes developing TRVs that consider measures of exposure and effects that could adversely impact populations of wildlife (for example, chronic studies on reproduction, growth, and survival). Mammalian studies on numerous sublethal endpoints (for example, systemic, immunological, developmental, respiratory, cardiovascular, gastrointestinal, ocular, hepatic) have been conducted for PFOS, PFOA, and other PFAS and are well described in the Toxicological Profile for Perfluoroalkyls (ATSDR 2021), but these are less commonly used for TRV development for ERAs. These sublethal, mostly systemic or organ function-based TRVs are really only used for ERAs in the absence of reproduction, survival, or growth data. Mammalian TRVs for the purposes of ERA can be developed for the majority of the Third Unregulated Contaminant Monitoring Rule (UCMR3) PFAS compounds listed in Section 8.2.2.2. Recommendations for selecting TRVs from available data for conducting ERAs are included in Divine et al. (2020), Conder et al. (2020), and Johnson et al. (2021). Avian oral dosing studies useful for ERA are less available. The dietary acute and chronic studies by Newsted et al. (2005); Newsted et al. (2007) examining PFOS exposure in mallard and bobwhite quail were the first published work relevant to ERA. Divine et al. (2020) and Conder et al. (2020) presented the data from their studies as well as data from other studies and for other PFAS that can be used for selecting avian TRVs. More recent publications relevant to ERA include that of Bursian et al. (2021), who looked at oral exposure of Japanese quail (Cortunix japonica) to PFOS, PFOA, and AFFF (both 3M and Ansul products), and (Dennis et al. 2020, 2021, 2021) who looked at northern bobwhite quail exposed to PFOS, PFHxS, and PFHxA. Newsted et al. (2005) and Molina et al. (2006) have also reported the results of bird egg injection studies using PFOS, while Cassone et al. (2012) and Norden, Berger, and Engwall (2016) have published in ovo studies with other PFAS. A caution with interpreting these egg studies is the uncertainty as to whether naturally accumulated concentrations have the same adverse effect as concentrations administered via injection in ovo. There also can be differences when measuring whole egg, yolk, or albumin (Custer, Gray, and Custer 2010). Finally, there is currently not enough data for modeling egg tissue concentration for these chemicals.
Reptiles are among the least studied vertebrate taxa in ecotoxicology (Hopkins 2000; Weir, Suski, and Salice 2010) despite contamination threatening reptile populations worldwide (Gibbons et al. 2000). A recent study (Furst, Weible, and Salice 2019) exposing brown anoles (Anolis sagrei) to PFOS and PFHxS provides data relevant to ERA. Measures of apical endpoints included decreased growth of juveniles exposed to PFOS, while exposure to PFHxS resulted in decreased egg viability in female anoles. To date, there are no other published reptile toxicity data available for any PFAS, although studies have shown PFAS tissue concentrations from some reptile species (Wang et al. 2013; Bangma et al. 2017).
Amphibian toxicity data are also limited, though more are available than are reptile data. Toxicity tests are available for eight different PFAS in the USEPA ECOTOX database and address exposure to four different species: the African clawed frog (Xenopus laevis), western clawed frog (X. tropicalis), Asiatic toad (Bufo gargarizans), and northern leopard frog (Rana pipiens)(USEPA 2019). A recent study (Flynn et al. 2019) is also available that looked at effects in American bullfrogs (Rana catesbeiana) exposed to a mixture of PFOS and PFOA. A laboratory bioaccumulation study (Abercrombie et al. 2019) of PFOS and PFOA in the eastern tiger salamander (Ambystoma tigrinum) and the American toad (Anaxyrus americanus) also provides some useful data for ERA.
For lower trophic-level organisms such as plants and invertebrates, toxicological data are typically generated through studies with direct exposure to spiked media. Studies are available to develop thresholds for use in ERAs, as has been done by ECCC (2018), CRC CARE (2017), Conder et al. (2020), and Divine et al. (2020). SSDs produced by CRC CARE (2017) showed lettuce to be more sensitive to PFOS than earthworms, but found the opposite occurred for PFOA. Divine et al. (2020) calculated Tier I water quality values for PFOS and PFOA, Tier II water quality values for another 21 PFAS, and soil screening values for plants and soil invertebrates for 6 PFAS each. Giesy et al. (2010) and ECCC (2018) generated PFOS SSDs for freshwater aquatic organisms, from which thresholds were derived. CRC CARE presented SSDs for PFOS and PFOA for marine waters and for soil to establish their thresholds. Giesy et al. (2010) noted that some guidelines for developing criteria from SSDs rely heavily on the four lowest effect concentrations; thus, results can be skewed if one genus or species is significantly more sensitive than others. In the freshwater SSD for PFOS generated by Giesy et al. (2010), Chironamous tentans (a species of midge) were 40 times more sensitive than the next most sensitive species, the fathead minnow (Pimephales promelas). However, the ECCC (2018) SSD does not show the same difference in sensitivity with a reported 14-day growth LOEC for Japanese rice fish (Oryzias latipes) below the C. tentans 10-day NOEC. For marine waters, fish are among the most sensitive organisms for both PFOS and PFOA as shown in SSDs (CRC CARE 2017), but they are more sensitive by just an order of magnitude or less. Additional studies by Simpson et al. (2021) and Hayman et al. (2021) have expanded the understanding of potential toxic effects in aquatic organisms in marine waters but an SSD that includes data from both of these studies has not been published.
SSDs have not been published for avian, mammalian, reptilian, or amphibian species. Although SSDs could possibly be generated for laboratory mammalian species exposed to some PFAS, data are insufficient to generate robust SSDs that are applicable to wildlife species. Mammalian SSDs would include mostly rat and mouse studies with a few monkey and rabbit studies. Extrapolation to other orders would be required. Existing data would be more conducive to an effects distribution because the number of species within the class of organisms would be so limited. A limited amount of published data is available for avian, reptilian, or amphibian animals, but these data are insufficient to determine a robust SSD or even an effects distribution.
Available toxicological data clearly do not adequately cover the range of organisms that are exposed to PFAS or within which PFAS have been detected, nor do the data have much breadth for chemicals beyond PFOS and PFOA. Sensitivity variation for aquatic organisms is evident from the SSDs, and likely sensitivity ranges for untested wildlife leave a clear knowledge gap for some or even most ERAs. However, this problem is not unique to PFAS. As with many other bioaccumulative and biomagnifying compounds, this knowledge gap can be addressed by using available data from surrogate organisms (for example, the closest taxonomic laboratory test species) and making some assumptions. The uncertainty in the potential difference in sensitivity needs to be acknowledged and discussed within ERAs. However, pending the outcome of quantitative analysis, risk conclusions and even risk management decisions are possible on a site-specific basis. Although extrapolations with surrogates is a common practice in ERA, caution should be used and decisions should be made in concurrence with regulatory agencies or other applicable stakeholders.
9.2.1.3 Ecological Toxicity of Mixtures
An additional major challenge in effects assessment for PFAS is considering the toxicity of mixtures. PFAS nearly always occur as complex mixtures in natural environments with multiple PFAS present at concentrations that vary by site and source of PFAS. At this time there are only limited data available to understand the toxicity of more than just a few chemicals based on single chemical exposure experiments such as direct toxicity to lower trophic level organisms or dietary exposures to upper trophic level wildlife. Thus, the ability to understand the toxicity of mixtures is limited by the scarcity of available toxicity data. However, in the absence of such data, several strategies using structural and physical properties and toxicity information from PFAS with available data may be helpful to infer potential mixtures effects, including mixtures with PFAS of unknown toxicity. These strategies include the use of in silico predictive techniques (for example, quantitative structure-activity relationships (QSARs)), using read-across from data-rich PFAS to inform data-poor PFAS, and estimating potential mixture risk using dose-addition risk assessment methods (for example, hazard index or relative potency factor approaches). Examples of published studies using QSAR for estimating PFAS toxicity include Hoover et al. (2019), Kovarich et al. (2012), and Cheng and Ng (2019) and additional citations within each of those publications. These studies typically evaluate an in vitro endpoint (such as cell death (Hoover et al. 2019)) or in silico measurements (such as the binding ability of known PFAS to specific cellular proteins (Cheng and Ng 2019; Kovarich et al. 2012) and then use QSAR to estimate how other PFAS would respond to that same endpoint or target. Similarly, Droge (2019) derived phospholipid membrane−water partition coefficients (KMW) that were used within a model to predict induction of narcosis (LC50,narc) and which could be used in the future with KMW-QSARs.
Most of these studies aim to fill data gaps for individual PFAS. Since it is not yet clear how PFAS may cause toxicity to various ecological receptors, the applicability of these data to ecological risk assessment of mixtures is unknown. However, with additional chemical-specific data, methods for estimated combined toxicity, such as hazard index or relative potency factors, could then be applied. Caution should be used when applying any of these approaches; some of these methods are novel and others may be well established but are predicated on specific data-supported assumptions that have not been universally accepted. Standardized toxicity tests, such as those performed with terrestrial and aquatic invertebrates and plants, are available for performing controlled dosing experiments and for exposure tests with field samples containing natural mixtures. Laboratory dosing experiments with whole mixtures, mixtures of prioritized PFAS, or with specific formulations, and with exposure concentrations that bracket environmental relevance, are all needed to inform ecological toxicity of mixtures.
There are a number of ongoing research projects investigating simultaneous exposure of ecological receptors to multiple PFAS (primarily the UCMR3 chemicals) and their precursors, with these studies mostly using binary mixtures (Lee et al. 2017; Flynn et al. 2019; Bursian et al. 2021; Dennis et al. 2020; McCarthy et al. 2021). However, the relative toxicity, additivity, or synergistic effects of PFAS remain incompletely understood and still uncertain. McCarthy, Roark, and Middleton (2021) discussed some of the challenges with designing, conducting, and interpreting toxicity tests with mixtures of PFAS. The understanding of PFAS mixtures is still developing and there is little consensus in the scientific community regarding how to assess the potential adverse impacts of PFAS mixtures. Complicating a limited understanding of PFAS mixture toxicity is that both absolute and relative concentrations of PFAS mixtures will vary across sites and across time. Reliance on empirical data such as site-specific toxicity sampling or community structure studies ultimately may be needed to fully understand the potential impacts of mixtures.
9.2.2 Ecological Exposure Assessment
Detections of PFAS in tissues of top predators within both aquatic and terrestrial ecosystems (Section 6.5) points to ongoing exposure from bioaccumulative and possibly biomagnifying PFAS (Section 5.5.3). Thus, accuracy and realism within exposure and risk estimates for PFAS are important to making informed risk management decisions. With the challenges of accounting for multiple exposure pathways, building strong food web and ecological exposure pathway models is an important foundation of PFAS ERAs. Once completed, these models can be used to identify the key receptors and measures of exposure to complete the assessments.
For aquatic ecosystems, published data from laboratory studies and specific field sites are available that include both BCFs, BAFs, and biota-sediment accumulation factors (BSAFs). These values, some of which are discussed and presented in Section 5 and Table 5-1 (provided as a separate Excel file), can be used to model the measures of exposure for aquatic ecosystems. (Larson, Conder, and Arblaster 2018) used such data to conduct food chain modeling in four different avian receptors. Published values for fish are common; however, to date these values are not standardized in how they are reported (for example, wet versus dry weight; organic carbon or lipid normalization). Most importantly, these data are highly variant (Table 5-1); Environment Canada (2006) reported that field BAFs for PFOS in Canadian biota range from 6,300 to 125,000. Burkhard et al. (2012) reported that within published data sources (Giesy et al. 2010; Houde et al. 2006), laboratory and field bioaccumulation metrics usually do not agree. According to Burkhard et al. (2012), field-generated BAFs (wet weight tissue to field water plus some ingestion) for PFOS exceed BCFs (wet weight tissue to lab water) predicted in the laboratory. This is undoubtedly due to the inability or inaccuracy of laboratory models to account for both direct and food ingestion exposure pathways. LaRoe et al. (2017) pointed out that laboratory values include only accumulation across the gill membrane. Thus, ERAs are challenged with attempting to address both pathways. Larson, Conder, and Arblaster (2018) demonstrated that using environmentally relevant sediment concentrations with standard food chain models with both BSAFs and BAFs suggested sediment pathways may be underrepresented and studied. Although the combination of direct and ingestion pathways is primarily a challenge for aquatic systems, assessing risk to wildlife exposed to multiple media (for example, amphibians, semiaquatic wildlife) is also problematic. As noted by Divine et al. (2020), there are differences between the numeric value of BSAFs developed from field data versus laboratory methods, and also between values from laboratory studies using the same methods.
In addition to fish, accumulation values for benthic organisms (California black worm, Lumbriculus variegatus, Higgins (Higgins et al. 2007) and (Lasier et al. 2011); oysters, Ostrea edulis, (Thompson et al. 2011) and pelagic invertebrates (D. magna), (Dai et al. 2013)) have also been reported. Example BSAF values from Lasier et al. (2011) for PFOS, PFOA, PFNA, PFBS, and PFHpA range from 7 to 49 kg sediment/kg tissue wet weight (as reported by authors). Divine et al. (2020) and Conder et al. (2020) summarized BSAFs for aquatic invertebrates and bivalves and for aquatic crustaceans for 15 PFAS. Both reports also discussed a variety of PFAS BAFs for amphibians, aquatic plants, benthic invertebrates, crustaceans, and fish. There is overlap, as well as difference, in the studies discussed and presented by Conder et al. (2020) and Divine et al. (2020). Much of this data is also described in Section 5.5. The Conder et al. (2020) report leans toward describing available data while the Divine et al. (2020) report goes a step further and attempts to apply the data to develop screening values from wildlife food web models, similar to the secondary poisoning values available in the EU (Table 9-6).
Data for terrestrial systems are limited to primarily plants (agricultural crops) and earthworms, with little available for vertebrate prey tissue. One exception is Müller et al. (2011), which published data for a soil-to-caribou-to-wolf BAF used by ECCC (2018) in establishing a soil threshold protective of terrestrial carnivores at 2.6 mg PFOS/kg soil. In nearly all cases, these BAFs and BSAFs are available only for PFOS, though the Lasier et al. (2011) study can be used to identify BSAFs for five of the six UCMR3 PFAS.
Caution should be used in applying any of the published bioaccumulation or biomagnification data for desktop exposure estimates that are in turn used to justify remedial action. Several factors and uncertainties are associated with performing desktop food chain modeling with the limited amount of published data. Some of these considerations include the following:
- differences in diets of receptors at investigation sites versus that of studies documented in the published literature: differences in the proportions of prey items; differences in the uptake and elimination rates of PFAS or overall bioaccumulation of PFAS by the prey
- differences in physiology between the site receptors and those in published literature: capacity and magnitude of transformation; metabolism and uptake and elimination rates of PFAS; the amount/composition of protein-containing tissues to which PFAS bind; species home range and migration
- differences in physiochemical properties of the abiotic media containing PFAS between investigation sites and published study sites: bioavailability and uptake of PFAS; environmental processes (photolysis, hydrolysis, microbial aerobic and anaerobic metabolism); the presence of precursors. There is not a sufficient set of bioaccumulation data to date to account for these variations. Such studies were part of the 2019 Statements of Need for Strategic Environmental Research and Development Program(SERDP) grant projects.
These uncertainties are not completely unique to PFAS, as there are many other contaminants for which risk assessments are performed. Though there is some uncertainty with desktop food chain models for PFAS based on abiotic media, quantitative modeling does not need to be avoided. Two conclusions should be reached through food chain modeling with abiotic media and literature based BAFs/BSAFs/BCFs: either concentrations at the site are sufficiently low such that it can be concluded that risk to the environment is negligible and acceptable or concentrations suggest further evaluation by either refined baseline problem formulation or a baseline ecological risk assessment (BERA). Conducting BERAs for sites with PFAS should not be substantially different from BERAs for sites with other chemicals. Either in situ or ex situ direct toxicity tests with representative organisms can and should be performed when exceeding the limited ecological risk thresholds that are available. Likewise, measured concentrations of PFAS in prey should be obtained if desktop food chain modeled exposure exceeds TRVs. But the biggest challenges for measuring PFAS in biota have to do with the unique analytical chemistry method issues (Section 11). Challenges such as selecting the correct biota to sample, matching the prey items to the diets of upper trophic level biota, or obtaining sufficient tissue volume for chemical analysis may exist, but these issues are not unique to PFAS investigations.
9.2.3 Risk Characterization
Some aquatic toxicity data are available for environmental risk assessment for a few PFAS, but wildlife data are still incomplete. Adequate, though not abundant, data are available for completing wildlife risk assessment, primarily for PFOS. The ability to complete risk assessments for other PFAS regularly analyzed and detected in environmental investigations (Section 6) is limited. However, with the exposure data discussed in Section 5.5 and Section 6.5, and methods discussed in Section 9.2.2, the foundations of a quantitative risk characterization can be completed for PFOS and to an extent, PFOA. Risk assessment for other PFAS can be made with some conservative assumptions and use of PFOS data as a surrogate. The ability to combine effects thresholds (Section 7.2) and exposures to characterize risk to environmental receptors is outlined in a few publicly available sources. McCarthy, Kappleman, and DiGuiseppi (2017), Conder et al. (2020), and Divine et al. (2020) have identified exposure factors and effects thresholds that can be used for completing quantitative ERA within current regulatory frameworks.
The risk characterization of any chemical has uncertainty associated with it, and ecological effects characterization for PFAS is no exception. However, at this time based on the data presented in this section, meaningful PFAS risk management decisions can be supported in some situations using the current state of ecological risk assessment science. Broad risk management decisions regarding the ecological risk of PFAS should not be made for most risk assessments. In some cases, with consideration of the knowledge gaps and uncertainties for the site-specific scenarios being evaluated, stakeholders can work together to reach defendable scientific management decisions. Such risk characterizations using non-site-specific abiotic media, surrogate information, and tools can form the basis of screening level assessments. These screening assessments can be used to make more informed decisions regarding the need for site-specific assessments, including the collection of site-specific tissue data. However, within these screening assessments, discussion of the uncertainties and data gaps and assumptions made should be included to inform the risk management decisions.
9.3 Uncertainty
In performing a site risk assessment, including information and a discussion regarding key factors of uncertainty in the risk characterization can be important. As noted by USEPA (1989), the source and degree of uncertainty associated with the risk characterization is needed to help decision makers (for example, risk managers, stakeholders), with sufficient level of detail to allow them to make informed risk management decisions (National Research Council 2009).
As noted throughout this guidance, while the science of characterizing and evaluating potential risks associated with PFAS exposure continues to develop, there are still uncertainties that arise in conducting site-specific risk assessments for sites with PFAS impacts. This section lists potentially critical uncertainties that, depending on the methodologies and assumptions used in a particular site-specific risk assessment, may warrant a discussion to help decision makers and stakeholders interpret and appropriately use the results of a risk assessment.
9.3.1 Fate and Transport
Site-specific risk assessments typically characterize risks associated with potential contaminant exposure that could occur currently or in the future. To characterize potential future exposures, conservative models are often used as tools to predict the fate and transport of chemicals in the environment. With regard to PFAS fate and transport, uncertainties can be introduced as follows:
- Estimating future environmental concentrations due to airborne wet and dry deposition (Section 5.3.2)
- Estimating the transformation of PFAA precursors to PFAA daughter end products (Section 5.4.2, Section 10.4.4) in the environment
- Modeling groundwater transport considering such factors as chemical-specific retardation (Section 10.4.1) and back-diffusion (Section 10.4.3.3)
- Estimating the bioaccumulation/bioconcentration of PFAS (Section 5.5.2, Section 9.2.1, Section 9.2.2) in a particular animal/plant or via food chain modeling
9.3.2 Human Toxicity
Human health risk assessments typically involve the use of toxicity values that are derived in a manner that is intended to represent a “reasonable conservative estimate” (USEPA 2012) of the dose-response in humans. Most of the toxicity values that have been derived by agencies for PFAS for use in site risk assessments are based upon animal studies, with human data used to support the hazard identification component of the risk assessment. However, it is noted that USEPA (2023, 2023, 2023), as well as CA OEHHA (2023) recently used human epidemiological data as the basis for draft noncancer toxicity factors (RfDs) for PFOA and PFOS and CSF for PFOA (Section 7.1.4). Additionally, there is a lack of toxicity values for many PFAS, which with their absence could result in an underestimation of the risks associated with PFAS exposure.
Overall, with regard to PFAS human toxicity, uncertainties in conducting a risk assessment can be introduced as follows:
- Missing dose-response information for site-related PFAS to which receptors could be exposed (Section 7.1, Section 9.1.1.2)
- Using toxicity values for a particular PFAS as a surrogate for another (Section 9.1.1.2)
9.3.3 Ecological Toxicity
As with human health risk assessments, ERAs often use TRVs that are generic and not site-specific. These generic TRVs are conservative by design because they are used for screening purposes (USEPA 2004). Likewise, there is a degree of conservatism incorporated into the derivation of generic criteria (for example, ambient water criteria) to account for uncertainty (Section 9.2.1).
Overall, with regard to PFAS ecological toxicity, uncertainties in conducting a risk assessment stem from using toxicological information from surrogate organism(s) to evaluate potential risks for organisms for which toxicity studies do not exist (Section 9.2.1)
9.3.4 Accounting for Nonsite-Related PFAS
Site-specific risk assessments rely on site characterization information (and as needed, modeling) to help estimate the amount of exposure receptors could be subject to currently or in the future. Given the widespread presence of PFAS in the environment (Section 6), including the potential of upgradient off-site PFAS impacts to migrate onto subject properties (Section 10.5), discerning “background” anthropogenic or off-site PFAS impacts at a site from site-related impacts can be challenging. To streamline risk assessments, it may be conservatively assumed initially that concentrations of PFAS are entirely site-related. Doing so, however, may overestimate the risks associated with site-related releases.
Updated September 2023.