2.6 PFAS Releases to the Environment
This section summarizes sources of PFAS releases to the environment that have the potential for significant environmental impact, based on the type and magnitude of the release, and the types and concentrations of PFAS associated with that release. These sources are sites where PFAS could be, or are known to have been, released to the environment, even if the site is not the location where the PFAS were generated or used. Refer to Section 2.1 for a discussion of the relative significance of releases and source control, as not all of these facilities will have, or have been documented to have, PFAS releases, and not all releases are of the same magnitude. “In the absence of high-quality testing data, PFAS contamination could be presumed” (Salvatore et al. 2022) at major sources and certain facilities as mentioned below. In addition, USEPA has developed an online database called the PFAS Analytic Tools that provides access to different sources of information about potential PFAS sources, drinking water sampling data, occurrence in environmental media, among others (USEPA 2023).
These major sources are located both in the United States and abroad, and include:
- industrial facilities that produce PFAS or process PFAS, or facilities that use PFAS chemicals or products in manufacturing or other activities (Section 2.6.1)
- areas where fluorine-containing Class B firefighting foams are stored, used, or released (Section 2.6.2)
- waste management facilities, such as landfills (Section 2.6.3)
- wastewater treatment residuals and areas of biosolids production and application, with more significant impacts associated with industrial wastewater discharges (Section 2.6.4).
- commercial inputs like schools, hotels, and box stores that can discharge high concentrations, linked to commercial cleaning activities like floor treatments (Section 2.6.1.7)
The fate and transport processes and distribution of PFAS in the environment are discussed in Section 5. Media-specific occurrence data are discussed in Section 6. Information about risk assessment, and human and ecological receptors is included in Section 9. Discussion of conceptual site model (CSM) components for each of the PFAS release categories listed above are included in Section 10.2.
2.6.1 Major Manufacturing and Industry Sources
Industrial source sites include primary and secondary manufacturing facilities. Primary manufacturing facilities are those where PFAS-containing products are synthesized and made into products or chemical feedstocks, or where PFAS are used as processing aids in fluoropolymer production. PFAS processing aids are not intended to be in the final product, but may be present at trace quantities (3M Company 2003; Buck et al. 2011).
Secondary manufacturing facilities may use fluoropolymers and PFAS-based materials produced at primary manufacturing facilities as part of industrial processes, such as the application of coatings to finished products. In some industrial settings, PFAS have been used for worker safety purposes, such as using 6:2 FTS or PFOS-based materials to suppress harmful mists during electroplating activities (Section 2.6.1.3).
USEPA (2021) includes PFAS manufacturers and PFAS formulators in the organic chemicals, plastics, and synthetic fibers (OCPSF) point source category, which includes a broad range of sectors, raw materials, and unit operations that may manufacture or use PFAS. PFAS manufacturers are defined as facilities that manufacture PFAS through electrochemical fluorination, fluorotelomerization, or other processes. PFAS formulators are defined as facilities that blend, convert, or integrate PFAS feedstocks with other materials to produce new commercial or intermediate products. USEPA (2021) identified six OCPSF PFAS manufacturing facilities and seven OCPSF PFAS formulators located in Illinois, Alabama, New Jersey, North Carolina, West Virginia, Minnesota, Pennsylvania, Ohio, Virginia, and Michigan.
PFAS composition and release mechanisms will vary for each facility. The composition of PFAS released from industrial facilities depends on the type of PFAS produced or used by the facility.
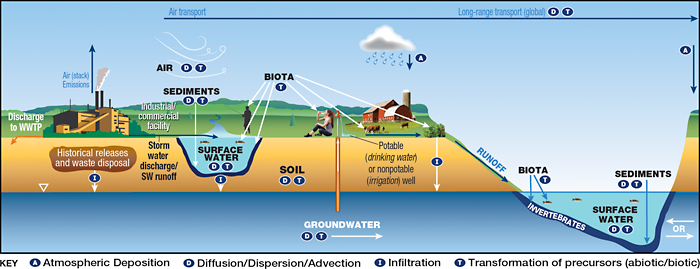
The general PFAS release mechanisms and pathways at industrial facilities are illustrated in CSM Figure 2-20 and include wastewater and stormwater discharges; on- and off-site disposal of solid wastes; accidental releases such as leaks and spills; and stack and fugitive emissions. Stack emissions may result in aerial deposition of PFAS to soil and surface water (with subsequent leaching and infiltration to groundwater) related to the facility (Davis et al. 2007; Shin et al. 2011), as well as short- and long-range air transport of PFAS. Industrial facilities may also contain areas where fire training or fire response using AFFF has occurred, AFFF storage areas, and AFFF fire suppression systems inside buildings. Like many AFFF release sites, industrial sites may also have releases of co-contaminants (solvents, petroleum products, etc.) that could potentially influence fate and transport of PFAS.
Figure 2-20. CSM for industrial sites.
Source: Adapted from figure by L. Trozzolo, TRC, used with permission.
The following subsections provide further details regarding potential sources of PFAS releases to the environment from PFAS use in manufacturing or industrial processes; these are not presented in order of the potential for significance of a release.
2.6.1.1 Building and Construction
Similar to other products, the chemical attributes of PFAS have led to advancements in building and construction materials. One particular application has been in composite wood and oriented strand board (OSB). Over the last 50 years, wood-based materials have used numerous additives for product strength and durability. A recent study performed on wood samples and OSB found primarily short-chain PFCAs and PFOA at concentrations ranging from 1.38 to 13.9 micrograms per kilogram (µg/kg) (Bečanová et al. 2016). Furthermore, wood fiber insulation has been shown to contain high amounts of PFHpA and other 5- to 8-carbon chain PFCAs (Bečanová et al. 2016). Many manufacturers use urea- or phenol-formaldehyde due to their performance and low cost; however, the composition of the resins used by many manufacturers is proprietary.
Other materials, including certain types of building insulation (phenolic foam) have shown high amounts of PFOS. Additionally, PFAS (predominantly C8–C20 gamma-omega-perfluorotelomer thiols with acrylamide) have been used in the production of lightweight concrete, concrete sandwich panels, and lightweight concrete blocks (Bečanová et al. 2016; Posner et al. 2013). The prevalence of these building materials in the construction of fire training areas, AFFF storage facilities, and other areas potentially exposed to PFAS led to potential issues with demolition waste. The porous nature of these materials (for example, concrete, brick) could lead to PFAS adsorption/absorption, representing a potential source of PFAS when disposed in landfills or recycling facilities (Australia Government DOD 2021).
PFAS, including fluoropolymers such as PTFE, are used in the manufacture of architectural fabrics, such as those used in the construction of roof domes, including large stadiums and transportation facilities (Performance Fluoropolymer Partnership 2021; Glüge et al. 2020).
PFOS-related chemicals have several uses in paint and varnishes. They can be used as wetting, leveling, and dispersing agents, and have also been used to improve gloss and antistatic properties. Additionally, they can be used as additives in dyestuff and ink. Furthermore, they can be used as pigment grinding aids or as agents to combat pigment flotation problems (KEMI 2004; RPA 2004). Fluorosurfactants are commonly used in coatings application for substrate wetting, leveling, reduction of surface tension, oil repellency, and dirt pickup resistance (Danish EPA 2015; Posner et al. 2013).
Information received from different suppliers within the paint and varnish industry suggests that fluorinated surfactants in general are much more expensive alternatives compared to other surfactants. Therefore, fluorosurfactants are used only for special purposes in paint and varnishes, where it is necessary to gain such a low surface tension that no other (nonfluorinated) alternatives can achieve (Danish EPA 2015).
Studies pertaining to PFAS in building materials continue to be published. The Green Science Policy Institute recently published a report that summarizes currently available studies for the purposes of informing those in the building and construction industry of the presence of PFAS and eliminating unnecessary uses (Fernandez, Kwiatkowski, and Bruton 2021).
2.6.1.2 Cable and Wiring
In the 1950s the wire and cable industry began to use extruded grades of PTFE. This is a suspension polymerization process, which does not require surfactants, unlike dispersion polymerizations (for example, Teflon-coated pans). Melt extrusion is the process by which most fluoropolymers are applied to wires. For instance, FEP, PFA, and PVDF are heated to 260°C and then melt extruded over wire to continuous lengths. The equipment used for melt-processable fluoropolymers requires temperature sensitivity of 427°F. PTFE is processed via paste extrusion for coating PTFE over wires due to its high melting point (ASTSWMO 2015; Kotthoff et al. 2015; Lau et al. 2007; Lindstrom, Strynar, and Libelo 2011; Oliaei et al. 2013; Renner 2001; Trudel et al. 2008). For more information on the safe handling of fluoropolymer resins during processing, see the Plastics Industry Association (2019) guidance document.
2.6.1.3 Metal Finishing and Plating
Electroplating is a process that uses electric current to apply a metal coating to the surface of an object. Metallic ions in an acidic electrolyte solution are used in the electrochemical deposition of metal coatings to the surface of the cathode (USEPA 1996).
PFAS, particularly PFOS, have been used as mist suppressants that are added to metal plating and finishing baths to prevent air emissions of toxic metal fumes. USEPA 2021 estimates that approximately half of the 1,339 chromium electroplating facilities in the United States still apply PFAS-based mist and fume suppressant. PFAS-containing chemicals may also be used in this industry as wetting agents, to reduce mechanical wear, and as surface coatings for reduced corrosion / enhanced appearance (USEPA 2021). Glüge et al. (2020) identified PFAS use in chrome, nickel, copper, tin, and zinc plating for lowering surface tension. In the United States, amendments to the National Emissions Standards for Hazardous Air Pollutants (NESHAP) under the Clean Air Act included a requirement to phase out the use of PFOS-based fume suppressants (a fume suppressant that contains 1% or greater PFOS by weight) in chromium electroplating by 2015 (USEPA 2012). Some countries (including the United States) have phased out the use of PFOS in some electroplating operations, adopting the use of other fluorotelomers (for example, 6:2 FTS) as a substitute in hard chrome plating operations (Danish EPA 2015; KEMI 2015) or changing decorative chrome plating operations to employ the less toxic trivalent chromium. PFAS known by the trade name F-53B have been used as metal plating mist suppressants in China (USEPA 2021; Bao et al. 2019). The toxicology of F-53B is reviewed in Section 17.2.6.1 (note that toxicological research may use different nomenclature for F-53B, namely 6:2 chlorinated polyfluoroether sulfonate (6:2 ClPFESA) and 8:2 ClPFESA (Munoz et al. 2019). Non-fluorinated fume suppressants are also now available.
Many different types of electroplating solutions can be used in plating activities, including hard and decorative chrome plating; chromic acid anodizing; nickel, cadmium, or lead plating; metal plating on plastics; and alkaline zinc plating. Chrome electroplating is the most significant contributor as it relates to PFAS use. In this process, PFAS are used as surfactants to reduce the surface tension of the electrolyte solution. Historically, PFOS was commonly used at a concentration of 5–10% to limit the development of bubbles and the emission of hexavalent chromium aerosols to workplace air, thereby reducing the potential hazard to workers posed by hexavalent chromium (USEPA 2009) (OSHA 2013) (Danish EPA 2015).
Studies show use of PFAS in these settings can result in high concentration wastewater discharges (USEPA 2009) and air emissions. Once the electrolyte solution can no longer be used, it may be treated to remove chromium and other metals, but PFOS and other PFAS may be present in effluent and deposited in sewage sludge (Danish EPA 2015). Investigations in Minnesota traced PFOS releases from one chrome plating operation to a wastewater treatment plant (WWTP) where elevated levels of PFOS were detected in the biosolids, effluent water, and fish in the receiving surface water (ATSDR 2008). Air emissions from another Minnesota chrome plater were found to have accumulated on the roof of the facility and from there contaminated stormwater and snow melting from the roof, which in turn contaminated the groundwater, a nearby surface water system, and fish (MPCA 2016). In another study in Minnesota looking at PFAS air and deposition monitoring, elevated PFAS were found in the vicinity of a chromium plater (MPCA 2022).
According to a study in Michigan by the Michigan Department of Environment, Great Lakes, and Energy (EGLE), 320 metal finishers that had a history of using fume suppressants were found to have PFOS in wastewater effluent. The report noted that 15% of metal finishers were discharging to WWTPs at concentrations greater than screening criteria (12 ppt PFOS) and 5% were discharging greater than 1,000 ppt PFOS (MI EGLE 2020). Of the metal finishers discharging PFOS above screening criteria, 89% used hexavalent and/or trivalent chromium in their current or past processes. Chrome platers in Michigan were determined to be in compliance with the NESHAP and many replaced PFOS with a fume suppressant containing 6:2 FTS. Some chrome platers did not use PFOS-containing chemicals to control fumes and have not been found to be sources of PFOS to WWTPs. Nearly half of the chrome platers regulated under the NESHAP used mechanisms other than chemical fume suppression. It was concluded that current effluent containing PFOS from facilities that have complied with NESHAP originates from historical use of PFOS-containing fume suppressants.
2.6.1.4 Industrial Surfactants and Fluoropolymer Production
PFAS have been, and currently are, instrumental as surfactants in industrial and commercial production. In the recent past, some information pertaining to specific surfactant uses of PFAS has become publicly available, though much information still remains unavailable. Most well documented is the historical use of PFOA as a processing aid in the manufacturing of PTFE, where APFO is used to help mix together the chemicals needed to combine units of tetrafluoroethylene (TFE) to make PTFE. Similarly, APFN, the ammonium salt of perfluorononanoic acid (PFNA), has also been used in the production of PVDF. PVDF polymers that are produced with the aid of APFN are sold in solid phase, with notable residual APFN concentrations (100–200 ppm) (Prevedouros et al. 2006).
Since the voluntary phaseout of PFOA and related PFAS chemistries, replacement chemistries such as ADONA and the GenX process chemicals are now used in the production of fluoropolymers.
The PFAAs used as polymerization aids may occur as impurities/residuals in some fluoropolymer products, as discussed in detail in Section 2.2.2.1
PFAS are also used in the manufacturing of plastics and fluoropolymers, rubber, and compression mold release coatings. These have applications in tubing, piping, drums, molds, and resins (Poulsen et al. 2005; Prevedouros et al. 2006).
2.6.1.5 Paper Products and Packaging
Since the 1960s, PFAS have been used as grease-proofing agents on food contact materials (FCM) to prevent oil, grease, and moisture from foods from leaking through the packaging. This includes coated paper and cardboard such as pizza boxes, microwavable popcorn bags, parchment paper, fast food wrappers, paper cups, pet food bags, and other items (Rao and Baker 1994; Hekster, Laane, and De Voogt 2003; Poulsen et al. 2005; Trudel et al. 2008; Buck et al. 2011).
The U.S. Food and Drug Administration (FDA) currently approves more than 90 unique monomer and polymer PFAS in FCMs (USFDA 2016). In January 2016, the FDA rescinded approval for three families of long-chain PFAS used in FCMs, but these had been voluntarily removed from the market in 2011. N-MeFOSE and N-EtFOSE were historically used to produce surface coatings for textiles and paper products (Zaggia and Ameduri 2012). PFAS currently used in FCM include polyfluorinated polyether-based polymers and shorter chain PFAAs (Wang, et al. 2015; Schaider et al. 2017). See Section 8.2 for additional information regarding a voluntary phaseout of 6:2 FTOH.
The most common PFAS detected in U.S. fast food wrappers include PFCAs (for example, PFOA and PFHxA), PFSAs (for example, PFBS), and fluorotelomer sulfonates (for example, 6:2 FTS) (Schaider et al. 2017). Six of 20 FCM tested were found to contain detectable levels of PFOA even though in 2011 U.S. manufacturers had voluntarily agreed to stop distributing FCM that were manufactured using PFOA via an FDA initiative. The methodology was not sensitive enough to detect if the PFAS were intentionally added to the packaging material or if they were attributed to unintentional background levels (Schaider et al. 2017). Refer also to Section 2.4.3 on the USEPA 2010/2015 PFOA Stewardship Program, which discusses the phaseout of PFOA and potential sources of PFOA that may remain in commercial and consumer products.
2.6.1.6 Photolithography/Semiconductor Industry
The semiconductor industry historically has used PFOS for their surface-active properties in the fabrication of imaging devices such as digital cameras, cell phones, printers, and scanners (Poulsen et al. 2005). Studies have shown semiconductor waste streams containing the PFAAs PFBS, PFHxS, PFOS, PFHxA, PFHpA, PFOA, PFNA, PFDA, PFUnA, and PFDoA (Lin, Panchangam, and Lo 2009). Similarly, in photolithography processes, PFOS has been used predominantly in applying top-layer antireflective coatings (TARCs), bottom antireflective coatings (BARCs), and etchants. Smaller quantities of PFOS and longer-chain PFAS have been used in wet etchants, film developers, cleaners, protective coatings, and color filters (SIA 2008), with ongoing uses permitted (Section 2.4).
2.6.1.7 Textiles, Leather, and Apparel (Including Carpet and Furniture)
Surface treatment of textiles, leather, carpet, and furniture upholstery with PFAS to make them stain, oil, and water repellent occurs both before (that is, at the factory) and after consumer acquisition for ongoing stain, oil, and water repellency (Prevedouros et al. 2006; Ahrens 2011; Herzke, Olsson, and Posner 2012). Aftermarket PFAS-containing stain-repellent products for carpets allow consumers to treat carpets and textiles at home (Renner 2001; Hekster, Laane, and De Voogt 2003). Losses to the environment can be related to dry cleaning and laundering activities (Poulsen et al. 2005; 3M Company 2000).
Home textiles, including furniture and carpeting, as well as aftermarket PFAS surface treatment products, are also sources of long-chain perfluorinated chemical exposure (Guo et al. 2009). Textile coating operations may use water-emulsion or powdered feedstocks that contain greater proportions of PFCAs compared to PFSAs (Lassen et al. 2015; Gremmel, Frömel, and Knepper 2016). According to California EPA (CalEPA) CalEPA 2018, pg. 12, “The PFAS polymers used in carpets, rugs, and other textiles can contain various amounts of mobile residual raw materials, impurities, or transformation products, including PFAAs and other PFAA precursors such as fluorotelomer alcohols (FTOHs) and perfluoroalkyl sulfonamide alcohols.” Releases to the environment could occur from disposal of carpet cleaning wastewater (CalEPA 2018). Physical degradation of some consumer products (such as PFAS-treated textiles and carpets, as well as paper) may be a source of PFAS in house dust (Björklund, Thuresson, and de Wit 2009). The weathering of certain textiles can also be the causes of potential releases of PFAS (Schellenberger et al. 2022; van der Veen et al. 2020).
It should be noted that many treated home textiles and carpets are now manufactured with alternatives to long-chain PFAS; however, these products can have a long useful life, making it possible that items previously treated with long-chain PFAS are still in use (Brooke and Nwaogu 2004). A 2009 study of over 100 consumer products conducted by the USEPA and Arcadis indicated that pretreated carpet, treated upholstery and textiles, as well as other floor treatments, are likely the largest source of PFAS receptor exposure in American homes (Guo et al. 2009).
Other studies have since shown nonpolymeric PFAS in leather samples and outdoor textiles to impart water, oil and stain resistance; applications include protective clothing, outerwear, footwear, umbrellas, tents, and sails (OECD 2013; Walters and Santillo 2006; Kotthoff et al. 2015). Durable water repellent (DWR) is a fabric surface finish that creates a protective barrier. It is typically added at the factory, but is also available to consumers for apparel maintenance (Brooke and Nwaogu 2004). The finishes/treatments are applied to materials in mills/tanneries and as aftermarket applications by professionals or do-it-yourself consumers as aqueous dispersions. In some aftermarket applications, they are applied as solutions in hydrocarbon-based or halogenated solvents (OECD 2013).
2.6.1.8 Other Potential Commercial or Domestic Sources of PFAS Releases to the Environment
There is the potential for everyday uses of PFAS to result in relatively smaller releases of PFAS to the environment. Of note, these may include, but are not limited to leaching from materials to media (for example, well construction and plumbing materials), discharges to on-site wastewater disposal systems from use of household products and cosmetics, discharges from car washing and waxing, and use of ski waxes (professional ski wax technicians may have significant inhalation exposures to PFAS (Nilsson et al. 2013). Snowmelt and surface waters (Kwok et al. 2013), as well as snow, soil, and groundwater (Carlson and Tupper 2020), near ski areas may have measurable PFAS impacts.
2.6.2 Class B Fluorine-Containing Firefighting Foams
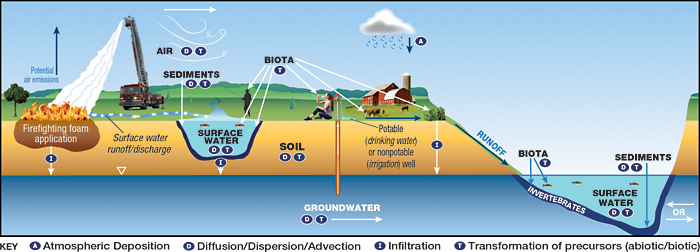
Some Class B firefighting foams designed for extinguishing flammable liquid hydrocarbon fires and vapor suppression may contain fluorine. These foams can be a major source of local PFAS release to the environment, with the CSM included in Figure 2-21.
Figure 2-21. CSM for fire training areas.
Source: Adapted from figure by L. Trozzolo, TRC, used with permission.
Class B firefighting foams are commercial surfactant solutions that have been (and continue to be) stored and used for fire suppression, fire training, and flammable vapor suppression at military installations and civilian facilities and airports (Hu et al. 2016), as well as at petroleum refineries and bulk storage facilities, and chemical manufacturing plants and storage facilities (CONCAWE 2016). Additionally, local fire departments in communities have used and may maintain quantities of firefighting foam in their inventories for use in training and emergency response. Facilities that manufactured firefighting foams and landfills that received firefighting waste are also potential sources. Refer to Section 3 for more detailed information about firefighting foams.
2.6.3 Solid Waste Management Facilities
Environmental releases associated with the use of PFAS-containing products are primarily related to management of solid waste (for example, disposal of used items in a landfill or other legacy disposal areas). Other solid waste facilities, such as scrap yards and metal salvage facilities, might also be a potential source of release to the environment. Some PFAS are considered hazardous waste by some states (Section 8). Additional information pertaining to disposal of PFAS and PFAS-containing materials at MSW landfills can be found in the 2020 USEPA Interim Guidance on the Destruction and Disposal of Perfluoroalkyl and Polyfluoroalkyl Substances and Materials Containing Perfluoroalkyl and Polyfluoroalkyl Substances (USEPA 2020).
Landfills can be sources of PFAS because they are the ultimate repositories for PFAS-contaminated industrial waste, sewage sludge from wastewater treatment facilities, and waste from site mitigation, as well as for PFAS-bearing consumer wastes, such as goods treated with hydrophobic, stain-resistant coatings (Busch et al. 2010; Eggen, Moeder, and Arukwe 2010). But the type and concentration of PFAS vary greatly among landfills, due to variations in the waste streams. Industrial waste can be a significant source of PFAS in landfills (as well as in wastewater and biosolids), particularly those that accept waste from facilities involved in the production or application of PFAS (Oliaei et al. 2013). Although MSW will contain PFAS due to its presence in so many consumer products, it generally is expected to have lower concentrations than landfills that accept industrial waste. Given the production timeline of PFAS, industrial, commercial, and consumer products and waste disposed since the 1950s are potential sources of PFAS release to the environment. As PFAS manufacturing processes change with time, the resulting type and composition of waste streams also change. PFAS production and use began several decades before the enactment of federal and state regulations governing waste disposal; as a consequence, environmental and drinking water impacts from disposal of legacy PFAS industrial and consumer waste have been documented (Oliaei, Kriens, and Weber 2010; Shin et al. 2011; MPCA 2017).
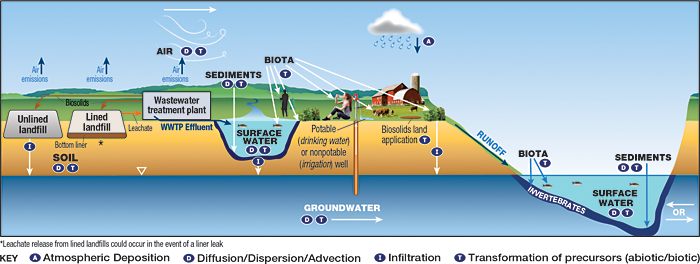
Figure 2-22 illustrates common elements of CSMs associated with the potential release scenarios at waste management facilities.
Figure 2-22. CSM for landfills and WWTPs.
Source: Adapted from figure by L. Trozzolo, TRC, used with permission.
2.6.3.1 Landfill Construction
Landfills are either lined or unlined (Figure 2-22). Municipal solid waste (MSW) landfills constructed since the 1990s are required by federal or state regulations to install a composite liner, a layer of compacted soil, and a leachate collection system (40 CFR 258.40). Although some states may have implemented construction standards at an earlier date, most landfills constructed before the 1990s were not required to have synthetic flexible membrane liners, compacted soil liners, or leachate collection systems, causing waste to be in direct contact with underlying soil or groundwater. Hazardous waste (Subtitle C) landfills have similar design requirements for the liner system, though an additional composite layer and leak detection layer are required (40 CFR 264.301). Construction and demolition (C&D) landfills or nonmunicipal solid waste landfills are subject to the requirements specified in 40 CFR 257 Part A (and if they intend to accept very small quantity generator waste, they are also subject to 40 CFR 257 Part B). Minimum design criteria for landfill liners are not specified in 40 CFR 257. Therefore, new C&D and nonmunicipal solid waste landfills may be permitted and constructed (or new cells added to existing facilities) without synthetic liners. Some states may have more restrictive requirements. Therefore, unlined landfills (and legacy disposal areas not classified as landfills) have a higher potential of contributing PFAS to groundwater (Oliaei et al. 2013). Properly constructed and operated modern landfills provide one of the few available disposal/management options for PFAS-containing waste, including wastewater solids, remedial/treatment waste, and consumer products. The USEPA Interim Guidance on the Destruction and Disposal of Perfluoroalkyl and Polyfluoroalkyl Substances and Materials Containing Perfluoroalkyl and Polyfluoroalkyl Substances provides further discussion about the use of landfills for management of PFAS-containing wastes, the potential for PFAS to be released to the environment from landfills, and the additional research and data that are needed to further assess the effectiveness of managing PFAS discharges and emissions from all landfills (USEPA 2020).
Landfills are currently required to use a daily cover or alternate daily cover. It is acceptable for alternative daily cover to include materials such as sludge, sludge-derived products, shredded automotive parts, spray-on foams, and other materials (Pohland and Graven 1993) that are possible sources of PFAS. Landfill caps reduce infiltration of water to waste and may reduce the overall mass of PFAS entering the environment from a landfill, but more research on their effectiveness is needed (Hamid, Li, and Grace 2018).
Leachate from some MSW landfills has been shown to be a source of PFAS release to the environment (Busch et al. 2010; Eggen, Moeder, and Arukwe 2010), although the fate and transport processes for PFAS through landfills into leachate are not well understood at this time. The processes for managing leachate have implications on the ultimate fate and transport of PFAS. If landfill liners or leachate collection systems fail, PFAS may directly enter the environment. Leachate collected from landfills is typically treated on site or transported to either a WWTP or evaporation ponds. Modern landfills with properly constructed and operated liner and leachate collection systems should generally protect the underlying groundwater from PFAS releases. Leachate treatment by WWTPs is common prior to discharge to surface water or distribution for agricultural or commercial use (Lang 2016). However, standard WWTP technologies are generally ineffective at reducing or eliminating PFAS (Hamid and Li 2016; Ahrens et al. 2016; CRC CARE 2017). As a result, the discharge of landfill leachate, even if treated at WWTPs, can be a significant source of release of some PFAS to the environment (Ahrens et al. 2016; CRC Care 2017). Although landfill leachate PFAS concentrations can be relatively high, landfill leachate discharged to WWTPs for treatment generally is considered a relatively minor source to the environment because the volume of leachate generated annually and sent to a WWTP for treatment is low compared to the flow volume in most WWTPs (Busch et al. 2010; Masoner et al. 2020; MWRA 2019). On a site-specific basis, the impact of leachate on combined wastewater influent PFAS mass loading can vary depending on the relative volumetric contribution of leachate to combined influent flows and the nature of the landfilled waste materials (Masoner et al. 2020). Furthermore, USEPA’s Effluent Guidelines Program Plan, (USEPA 2023) states “EPA evaluated discharge data from over 200 landfills from across the country and found PFAS present in the leachate at over 95 percent of the landfills. PFAS detections included 63 different PFAS with average concentrations for an individual compound as high as 14,000 parts-per-trillion (ppt).” However, in a limited study of five wastewater treatment facilities in Florida, Masoner et al. (2020) did not identify differences in the effluent PFAS loading of wastewater treatment facilities that received landfill leachate relative those that did not, suggesting the PFAS load present in wastewater treatment effluent is attributable to numerous sources. A similar finding was noted by MWRA (2019), though only PFOA and PFOS were investigated.
As part of EPA’s final Effluent Limitations Guidelines Plan 15, USEPA (2023) announced its plans to proceed with a rulemaking to address PFAS discharges from landfills; refer to Section 16.6 for more information on this rulemaking.
2.6.3.2 Waste Age
Landfills containing sources of PFAS may continue to release PFAS to leachate at slow but relatively steady rates for decades following initial placement. In modeled anaerobic landfill reactors, most of the release is attributed to biological, not physical, mechanisms, indicating that the low solubility of the compounds is not solely responsible for slow release rates from landfills (Allred et al. 2015; Lang et al. 2016). Legacy industrial waste landfills may constitute a major source of PFAS release to the environment (ATSDR 2008, 2012).
2.6.3.3 PFAS Composition
PFAS composition and concentration in leachates vary depending on waste age, climate, and waste composition (Allred et al. 2015; Lang et al. 2017). Relative concentrations of PFAS in leachate and groundwater from landfills are different from those at WWTPs and AFFF-contaminated sites. PFAS with fewer than eight carbons tend to dominate landfill leachate because they are less hydrophobic and therefore more likely to partition to the aqueous phase (Huset et al. 2011; Higgins and Luthy 2007). In particular, 5:3 fluorotelomer carboxylic acid (FTCA) is a common and often dominant constituent of PFAS found in landfills and is released from carpet in model anaerobic landfill reactors. This compound could prove to be an indicator of PFAS in the environment originating from landfills (Lang et al. 2017, 2016).
PFAS may also be released to the air from landfills, predominantly as fluorotelomer alcohols (FTOHs) and perfluorobutanoate (PFBA). In one study, total PFAS concentrations were 5–30 times greater at landfills than at background reference sites (Ahrens et al. 2011). PFAS release rates vary with time for a given waste mass, with climate (for example, rainfall) as the apparent driving factor for the variations (Lang et al. 2017; Benskin et al. 2012). Gas collection systems commonly employed at modern landfills should reduce possible PFAS emissions to air.
2.6.4 Wastewater Treatment and Wastewater Treatment Residuals and Biosolids
Consumer and industrial use of PFAS-containing materials, including disposal of landfill leachate and firefighting foam, may discharge PFAS-containing wastewater to municipal and industrial WWTPs (Lin, Panchangam, and Lo 2009; Ahrens et al. 2009) private septic systems, or other wastewater disposal systems.
2.6.4.1 Wastewater Treatment
WWTPs receive PFAS from a host of sources conveyed thru and aggregated in effluent and sludges, which can provide the following pathways for PFAS releases to the environment (Figure 2-22):
- point source discharges of effluent
- leakage or unintended releases from surface impoundments and structures
- air emissions
- management (for example, land application) and disposal (for example, landfilling, incineration) of biosolids (https://www.epa.gov/biosolids), wastewater solids and sludges, and other byproducts generated during the treatment process (Section 2.6.4.2). Refer to Section 12 for more information on incineration.
The composition of PFAS in these media is a function of the different sources to the WWTP influent and the WWTP processes (Chen, Lo, and Lee 2012; Oliaei, Kessler, and D. Kriens 2006; Frömel et al. 2016; Schultz et al. 2006), including:
- type and concentration of PFAS received by the WWTP
- biological and chemical transformation of polyfluorinated substances (that is, precursor PFAS) to intermediate and terminal transformation products, such as PFAAs
- physical or chemical partitioning, or both.
In addition to PFAAs, fluorotelomer sulfonates, and sulfonamides, another family of PFAS precursor compounds, polyfluoroalkyl phosphate monoesters (PAPs) and polyfluoroalkyl phosphate diesters (diPAPs) (particularly 6:2 and 8:2 PAP; and 6:2 and 8:2 diPAP) have been documented in wastewater treatment plant (WWTP) samples and especially biosolids (Eriksson, Haglund, and Karrman 2016; Fredriksson et al. 2022; Kim Lazcano et al. 2020; Lee et al. 2014). This family of PFAS precursors plays an important role since they are typically found at much higher concentrations than commonly measured PFAS (Aro et al. 2021; Fredriksson et al. 2022; Moodie et al. 2021; Schaefer et al. 2022) and they have been shown to biotransform to PFAAs (Eriksson, Haglund, and Karrman 2016). Fredriksson et al. (2022) reported diPAPs accounting for 66% of the total concentrations of PFCAs precursors in sludge samples with the sum of diPAPs reaching up to 632 µg/kg, consistent with findings by Schaefer et al. (2022). As a result of precursor biotransformation, the concentrations of PFAAs can increase from influent to effluent, while the concentrations of these PFAS precursors decrease (Chen et al. 2018; Dauchy et al. 2017; Houtz, Wang, and Park 2018).
PFAS occurrence is reported in small municipal wastewater treatment plants with only domestic sources (Sinclair and Kannan 2006). PFAS in non-industrial wastewater is attributed to environmental transformation of polyfluorinated microfibers that might be released by different sources, for example, water-resistant clothing during laundry (Schellenberger et al. 2019), food packaging (Choi et al. 2019; Schaider et al. 2017), human excretion after oral exposure (Ma, Hongkai, and Kurunthachalam 2020; Worley et al. 2017), and tap water (Andrews and Naidenko 2020; Filipovic and Berger 2015).
Thompson et al. (2022) suggested that domestic wastewater accounts for most of the PFAS load in WWTPs while historically industrial discharges was believed to cause the majority of the PFAS releases from WWTP.
Conventional sewage treatment methods used in WWTPs do not efficiently remove PFAAs (Ahrens et al. 2011; Schultz et al. 2006). Even WWTPs with advanced treatment technologies (such as granular activated carbon (GAC), powdered activated carbon (PAC), or reverse osmosis (RO)) may not fully remove all PFAS if these systems were not designed with the intent to remove PFAS in addition to other targeted contaminants. Some PFAAs are frequently detected in WWTP effluent (for example, PFOA and PFBS), with concentrations of some PFAS up to hundreds of ng/L (Tavasoli et al. 2021). Ahrens et al. (2011) and Hamid and Li (2016) suggested that WWTP effluent can be a major source of PFAAs to surface waters.
Evaluation of full-scale WWTPs has indicated that conventional primary (sedimentation and clarification) and secondary (aerobic biotransformation of organic matter) treatment processes can change PFAS concentrations and subgroups. For example, studies have shown increased concentrations of PFAAs in effluent, presumably from transformation of precursor PFAS (Schultz et al. 2006), and the possible creation of PFAAs from the oxidation of polyfluorinated precursors during the treatment process (Oliaei, Kessler, and D. Kriens 2006; Frömel et al. 2016; Houtz, Wang, and Park 2018).
PFAS may be concentrated in wastewater solids (for example, sewage sludge) generated throughout the wastewater treatment process (Schultz et al. 2006). PFAS may also be present in septage (solids removed from septic systems). Depending on waste management and disposal practices, land application or landfill disposal of wastewater solids, biosolids, or septage could potentially contaminate the environment.
Hu et al. (2016) suggested that the presence of WWTPs in an area could be predictive of the presence of PFOS and PFOA in drinking water. PFOS and PFOA are two of the most frequently reported PFAS in wastewater (Hamid and Li 2016) in addition to other relevant PFAS such as diPAPs (Schaefer et al. 2023 and Thompson et al. 2023); see Section 6.2.3 for more information. Using WWTP effluent-impacted surface water as a source of drinking water can, in turn, recycle the PFAS back to the WWTP, recirculating PFAS in the water cycle (Hamid and Li 2016).
At some WWTPs, studies have shown concentrations of PFAS in ambient air at WWTPs to be 1.5–15 times greater than background reference sites (Hamid and Li 2016). Hamid and Li (2016) noted that these elevated air concentrations of total PFAS include polyfluoroalkyls and that this has important implications considering the potential for their long-range transport and subsequent transformation to recalcitrant PFAAs. PFAS distribution (primarily PFAAs and FTOH, with higher concentrations of FTOH) changes based on the specific PFAS sources in the effluent and the type of treatment methods employed at the WWTP.
Michigan EGLE has a program for evaluating PFAS in WWTP influent, effluent, and biosolids including conducting studies to better understand the fate and transport of PFAS from land application of biosolids (MI EGLE 2021). This included sampling of WWTP influent, effluent, and associated sludge or biosolids at 42 WWTPs and sampling of soils, groundwater, and surface waters at eight biosolids land application sites. The land application sites that received biosolids with “typical” levels of PFAS generally exhibited non-detectable to low concentrations of PFAS in soils, surface waters. The sites that received biosolids with higher PFAS levels that EGLE considered to be “industrially-impacted” showed higher concentrations of PFAS in the soils, surface water and groundwater with some results exceeding certain Michigan criteria. Results from the broad biosolids sampling were used to develop an Interim Biosolids Land Application PFAS Strategy (MI EGLE 2022), which in conjunction with the other State initiatives, allowed the majority of WWTPs to maintain the option to land apply biosolids while maintaining protectiveness. Restrictions were placed on land application of biosolids containing PFAS above certain criteria. Additional information about the MI EGLE biosolids programs are linked from their web site (https://www.michigan.gov/egle/about/organization/Water-Resources/biosolids/pfas-related).
Human exposure to PFAS can occur from wastewater through leaching into groundwater from biosolids land application or landfill operations, bioaccumulation into food or unplanned/planned potable reuse (Ahrens et al. 2011; Glover, Quinones, and Dickenson 2018; Hu et al. 2016; Lindstrom et al. 2011). More information about human exposures to PFAS is included in Section 9.1.2.
2.6.4.2 Biosolids Production and Application
Biosolids or “sewage sludge” are products of liquid separation from solids in wastewater treatment systems which undergo further physical chemical treatments to produce nutrient-rich products. Biosolids are residuals that have been treated thermally and/or chemically to meet state and federal standards to allow beneficial reuse. Biosolids are managed through beneficial reuse with land application (agriculture, turf grass, land reclamation) or composting (fertilizer) or disposed of by landfilling, incineration, or other form of surface disposal (NACWA 2023). Biosolids must meet state and federal requirements prior to land application in agricultural and reclamation sites. Land application of biosolids can offer economic and waste management benefits to municipalities and farmers (Stulp 1995). It should be noted that biosolids are not wastewater solids, industrial-derived sludges, or sludges that are the untreated solids/sludge removed during the process of treating wastewater.
PFAS presence in wastewater treatment residuals generated at municipal WWTPs is the result of widespread presence in engineered and natural systems, including numerous consumer products and certain industrial processes. The residuals can contain varying levels of PFAS commensurate with sewer shed characteristics, one of which is the type of and degree of influence that industrial dischargers have on municipal WWTPs. Several studies have reported results of testing of biosolids for PFAS (MI EGLE 2022). PFAS (measured as PFCAs and PFSAs) have been found in domestic sewage sludge (Higgins et al. 2005; Yoo et al. 2009), and PFAS occurrence in biosolids is reported to be prevalent and nationwide (Venkatesan and Halsden 2013).
There are a number of methods currently used to manage biosolids. According to USEPA (2023), around 43% of biosolids are land applied (typically as agricultural fertilizer), 42% landfilled, 14% incinerated, and 1% are stored or deep-well injected in the United States. The National Biosolids Data Project (https://www.biosolidsdata.org/) similarly reports about half of the biosolids are land applied, 25% are disposed in landfills, and 15% managed through incineration. Given the significant proportion of biosolids land applied for agriculture, there is the potential for release of PFAS to the environment associated with biosolids production and application. A small number of states have placed restrictions on incineration of PFAS containing material until the efficiency of destruction is better understood through on-going studies. Landfilling biosolids may lead to increased PFAS in landfill leachate which must be managed or increase the potential for releases to groundwater or air. However, there may be a greater reliance on landfilling in some states as restrictions are made on other management options. Long term landfilling of biosolids would also stress existing landfill capacity. In addition, a small number of states have also restricted the land application of biosolids due to concerns related to PFAS.
Biosolids can be land applied in several ways, including subsurface injection of liquid biosolids, surface application of liquid biosolids, surface application of dewatered (semi-solid) biosolids, or application and incorporation of dewatered (semi-solid) biosolids. Once introduced to the environment through land application, PFAS may enter surface water through runoff or infiltrate to groundwater (Lindstrom, Strynar, and Libelo 2011). Biosolids are also dried/pelletized for direct use as a soil amendment/fertilizer and for use in fertilizer blends that are then marketed commercially. The potential effects on groundwater or surface water may depend on many factors including but not limited to the amount and composition of PFAS present in biosolids, soil properties, infiltration rate, land application practices, land use, precipitation, climate, and land slope. PFAS concentrations can be elevated in surface and groundwater in the vicinity of agricultural fields that received PFAS-contaminated biosolids over an extended period of time (Washington et al. 2010). The Washington et al. study was completed in an area that received industrial wastewater discharges from several PFAS-related industrial dischargers. Other studies indicate that the potential PFAS releases from municipal biosolids (for example, those generated at WWTPs that do not receive significant inputs from industries linked to PFAS), may still impact water quality, but at an apparent lower relative impact than at the industrial-influenced biosolids application sites (Gottschall et al. 2017). Pepper et al. (2021) examined PFAS in soil in Arizona following long-term application of biosolids. The study concluded “long-term land application resulted in low incidence of soil PFAS” and “PFAS soil concentrations in irrigated agricultural plots with or without land application of biosolids were similar” (Pepper et al. 2021).
The most abundant PFAS found in biosolids (PFOS and PFOA) are the same as those found in WWTP effluent, although biosolids may also contain other long-chain PFAS (Hamid and Li 2016; Washington et al. 2010). Although multiple studies have reported statistically significant data showing transformation of polyfluorinated substances to PFAAs in land-applied biosolids (Yoo et al. 2010; Sepulvado et al. 2011; Washington et al. 2010), other evidence indicates that some polyfluorinated substances remain in biosolids-amended soils for many years to decades (Yoo et al. 2010; Rich et al. 2015; Washington et al. 2018).
Application of municipal or industrial biosolids as a soil amendment can result in a transfer of PFAS to soil (Sepulvado et al. 2011). These PFAS can then be available for uptake by some plants and soil organisms (Yoo et al. 2011). There are indications that PFAAs can enter the food chain through the use of biosolids-amended soil (Lindstrom, Strynar, and Libelo 2011; Blaine et al. 2013; Blaine et al. 2014; Navarro et al. 2017). It is noted, however, that PFAAs present at one municipal biosolids application site were not found in grain grown in the application plot (Gottschall et al. 2017). Hamid and Li (2016) suggested that short-chain (< C7) PFAAs in biosolids subsequently used in land applications can lead to contamination of food (Section 5.6). Significant data gaps exist regarding the fate and transport mechanisms associated with land application of PFAS impacted biosolids and the associated impacts to the food chain are an active area of study (see Section 6.5).
Refer to Section 2.7.4.1 for information on Michigan EGLE’s program for evaluating PFAS in WWTP influent, effluent, and biosolids including conducting studies to better understand the fate and transport of PFAS from land application of biosolids (MI EGLE 2021). The Environmental Council of the States (ECOS) collected information in November 2022 from state environmental agencies about management of biosolids and published a report of their findings (ECOS 2023).
Updated September 2023.